A method for separating and determining Apremilast and its enantiomers by liquid chromatography
A technique of enantiomer and liquid chromatography, which is applied in the field of separation and determination of apremilast and its enantiomers by liquid chromatography, can solve the problems of peak shape difference, apremilast and its corresponding isomers Effective separation, low column efficiency and other problems, to achieve the effect of good peak shape, controllable quality assurance and high column efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Instruments and Conditions
[0037] American Agilent 1100 liquid chromatograph and Instrument workstation; automatic sample injection; instrument: use CHIRALPAK AS-H (5μm, 250×4.6mm) as the separation column; use n-hexane-methanol-isopropanol (volume ratio 100: 900:100) mobile phase; detection wavelength: 230nm; flow rate: 1.0ml / min; injection volume: 10μl; column temperature: 35°C.
[0038] Experimental steps:
[0039] Weigh 100.57 mg of Apremilast working reference substance and 100.79 mg of enantiomer reference substance, put them in 10ml measuring bottles respectively, dissolve them with acetonitrile and dilute to the mark, shake well, accurately measure 0.5ml, put them in 100ml measuring bottles respectively , diluted to the mark with the mobile phase, shaken up, as the stock solution of Apremilast and the stock solution of the enantiomer, respectively accurately measure 1.0ml and place it in a 10ml measuring bottle, dilute to the mark with the mobile phase, shake...
Embodiment 2
[0046] Determination of apremilast enantiomers by liquid chromatography.
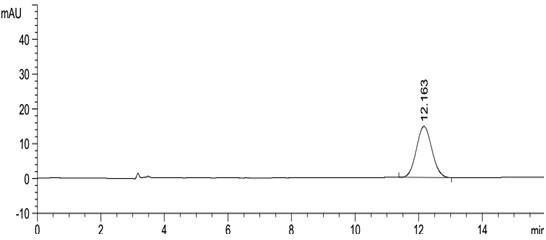
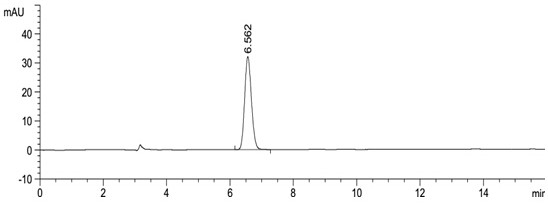
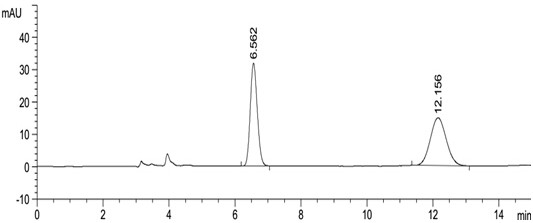
[0047] Take about 10 mg of this product, weigh it accurately, put it in a 10 ml measuring bottle, add 2 ml of acetonitrile to dissolve, dilute to the mark with mobile phase, shake well, and use it as the test solution. Precisely measure 0.5ml, put it in a 100ml measuring bottle, dilute to the mark with mobile phase, shake well, and use it as the reference substance solution. Carry out liquid chromatography analysis according to the chromatographic condition of embodiment 1, need testing solution except solvent peak and system peak, if there is enantiomer at about 0.54 place of apremilast relative retention time, calculate according to self-control method Its content, if there is no chromatographic peak at the relative retention time of about 0.54, there is no need to calculate the content. see results Figure 5 , Image 6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com