A preparing method of black single brookite phase titanium dioxide
A technology of titanium dioxide and brookite, applied in the direction of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc., can solve the problems of harsh synthesis conditions, multi-steps, limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
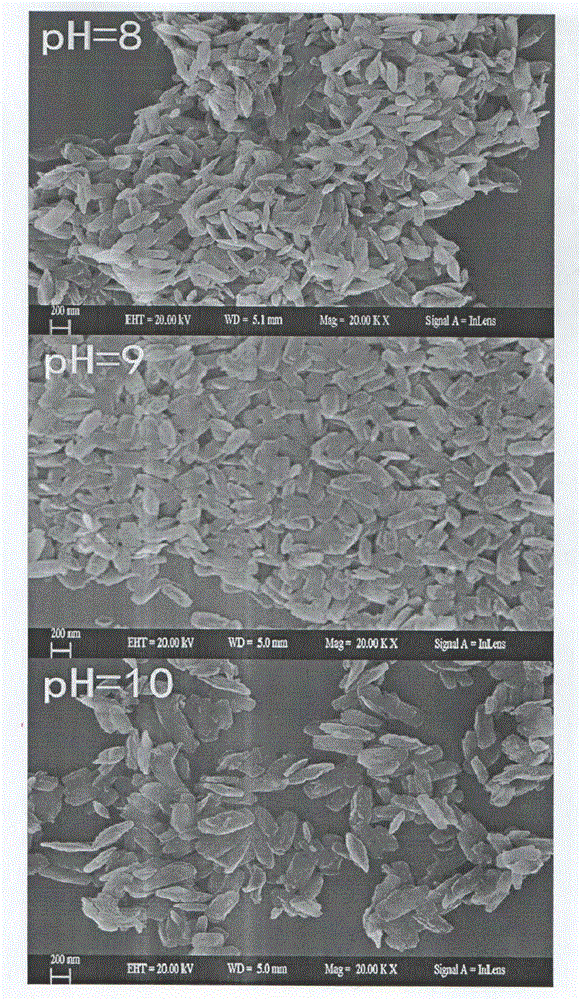
[0018] a. Dissolve 0.32g of titanium hydride in 2.5mL of deionized water, add 30mL of hydrogen peroxide with a mass fraction of 30% dropwise under constant stirring, and keep stirring the mixed reaction solution rapidly at room temperature for 12 hours to obtain a gel-like mixture;
[0019] b. Under uniform stirring, add 50 mL of deionized water to the mixture obtained in step a, adjust the pH of the mixed solution to 8 with 1.0 M sodium hydroxide solution, then add 0.4 g of sodium borohydride to obtain a transparent yellow mixed solution;
[0020] c. Place the mixed solution obtained in step b in an autoclave at a temperature of 180° C. and react for 24 hours;
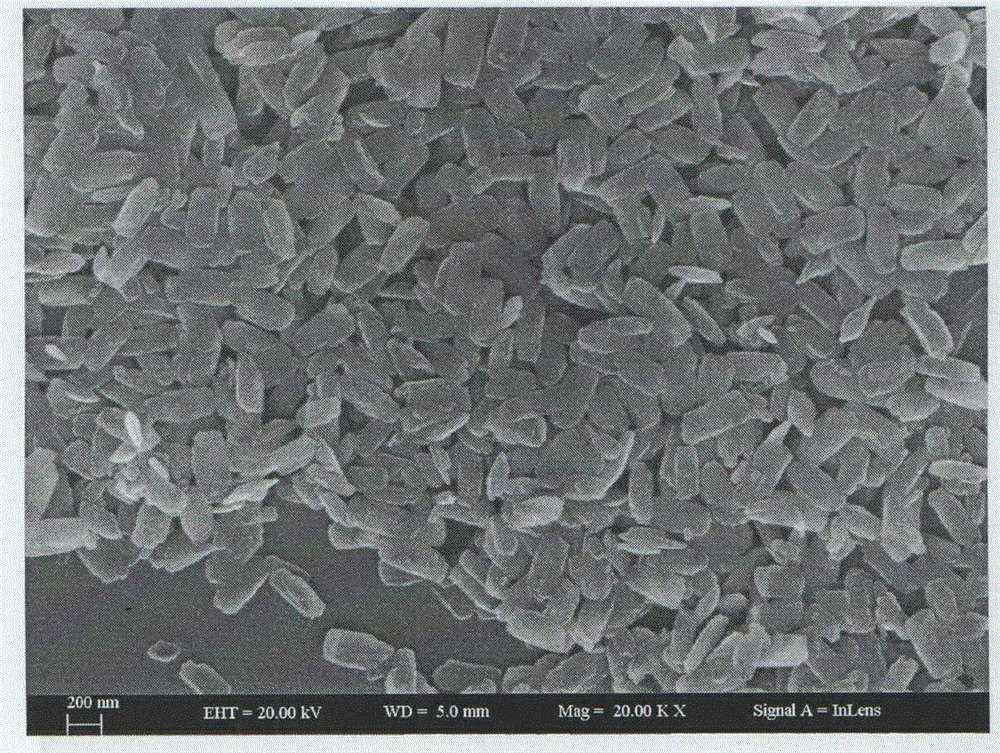
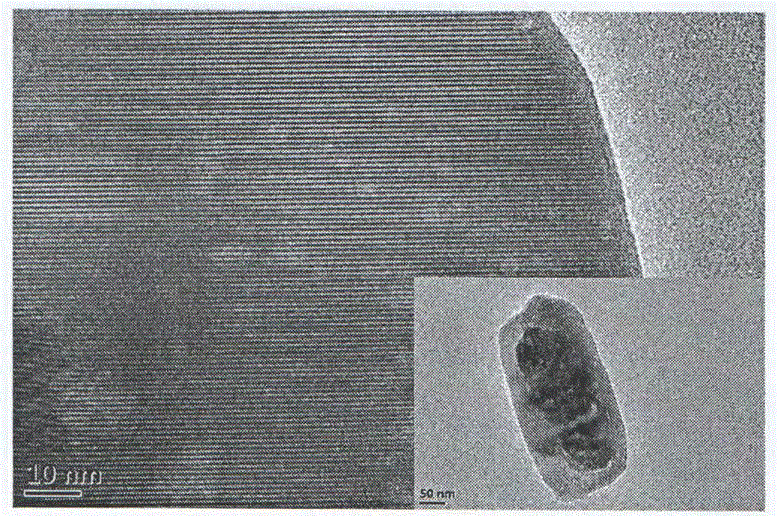
[0021] d. Remove the mixed solution obtained in step c from the autoclave, wash it in 50.0mL of 1.0M hydrochloric acid solution for 10h, and then centrifuge to obtain the precipitate, wash it twice with absolute ethanol, wash it three times with deionized water, and place it in Dry in a vacuum freeze-drying oven for 1...
Embodiment 2
[0024] a. Dissolve 0.32g of titanium hydride in 2.5mL of deionized water, add 30mL of hydrogen peroxide with a mass fraction of 30% dropwise under constant stirring, and keep stirring the mixed reaction solution rapidly at room temperature for 12 hours to obtain a gel-like mixture;
[0025] b. Under uniform stirring, add 50 mL of deionized water to the mixture obtained in step a, adjust the pH of the mixed solution to 9 with 1.0 M sodium hydroxide solution, then add 0.4 g of sodium borohydride to obtain a transparent yellow mixed solution;
[0026] c. Place the mixed solution obtained in step b in an autoclave at a temperature of 180° C. and react for 24 hours;
[0027] d. Remove the mixed solution obtained in step c from the autoclave, wash it in 50.0mL of 1.0M hydrochloric acid solution for 10h, and then centrifuge to obtain the precipitate, wash it twice with absolute ethanol, wash it three times with deionized water, and place it in Dry in a vacuum freeze-drying oven for 1...
Embodiment 3
[0030] a. Dissolve 0.32g of titanium hydride in 2.5mL of deionized water, add 30mL of hydrogen peroxide with a mass fraction of 30% dropwise under constant stirring, and keep stirring the mixed reaction solution rapidly at room temperature for 12 hours to obtain a gel-like mixture;
[0031] b. Under uniform stirring, add 50 mL of deionized water to the mixture obtained in step a, adjust the pH of the mixed solution to 10 with 1.0 M sodium hydroxide solution, then add 0.4 g of sodium borohydride to obtain a transparent yellow mixed solution;
[0032] c. Place the mixed solution obtained in step b in an autoclave at a temperature of 180° C. and react for 24 hours;
[0033] d. Remove the mixed solution obtained in step c from the autoclave, wash it in 50.0mL of 1.0M hydrochloric acid solution for 10h, and then centrifuge to obtain the precipitate, wash it twice with absolute ethanol, wash it three times with deionized water, and place it in Dry in a vacuum freeze-drying oven for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com