A kind of preparation method and application of dysprosium doped blue phosphor powder
A blue phosphor, soluble technology, applied in the field of preparation of dysprosium-doped blue phosphor, can solve the problems of difficult preparation, luminous effect and luminous intensity reduction, and achieves simple process, good particle consistency and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
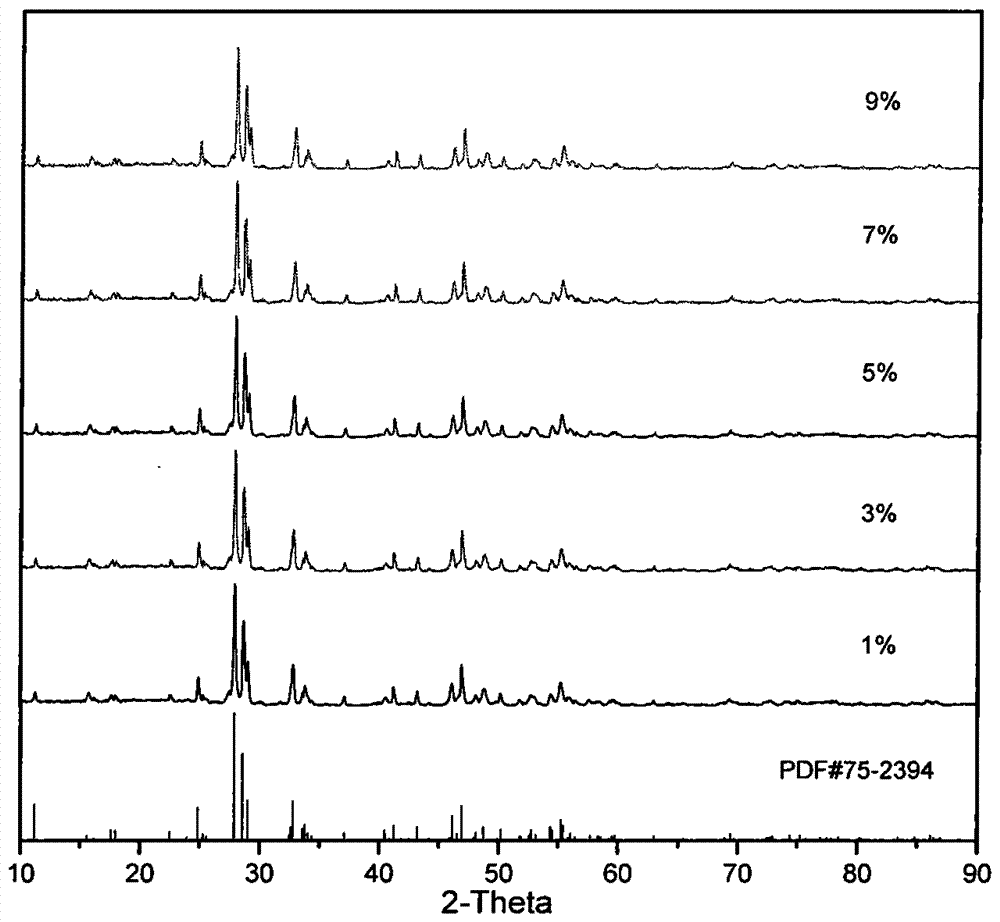
[0033] According to the general chemical formula: (1-x) La 2 o 3 -TiO 2 -xDy 2 o 3 (where x=(0.01-0.09) stoichiometric ratio weighs 2 (1-x) mmol lanthanum acetate (La(CH 3 COO) 3 , A.R.) was dissolved in an appropriate amount of distilled water, and 1 mmol of tetrabutyl titanate (C 16 h 36 o 4 Ti, A.R.), dissolved in 30ml of ethanol. According to the stoichiometric ratio, x=(1%, 3%, 5%, 7%, 9%) doped dysprosium acetate (Dy(CH 3 COO) 3 , A.R.), dropwise added 10mmol acetic acid (CH 3 COOH, A.R.), stirred for 1 h, and put into a water bath at 80°C for about 5 h to obtain a colloidal precipitate, the precursor sol C. The precursor sol C was taken out and placed in a corundum crucible, calcined in a muffle furnace at 1100° C. for 3 hours, and kept for 2 hours to obtain the required lanthanum titanate blue phosphor.
Embodiment 2
[0035] According to the general chemical formula: (1-x) La 2 o 3 -TiO 2 -xDy 2 o 3 (wherein x=(0.01-0.09)) stoichiometric ratio weighs 4 (1-x) mmol lanthanum nitrate (La(NO 3 )3 , A.R.) was dissolved in an appropriate amount of distilled water, and 2 mmol of tetrabutyl titanate (C 16 h 36 o 4 Ti, A.R.), dissolved in 50 ml methanol. According to the stoichiometric ratio, x=(1%, 3%, 5%, 7%, 9%) doped dysprosium nitrate (Dy(NO 3 ) 3 , A.R.), dropwise added 20mmol nitric acid (HNO 3 , A.R.), stirred for 1 h, put into a water bath at 100°C for about 4 h, and obtained a colloidal precipitate, the precursor sol C. The precursor sol C was taken out and placed in a corundum crucible, calcined in a muffle furnace at 1200° C. for 3 hours, and kept for 2 hours to obtain the required lanthanum titanate blue phosphor.
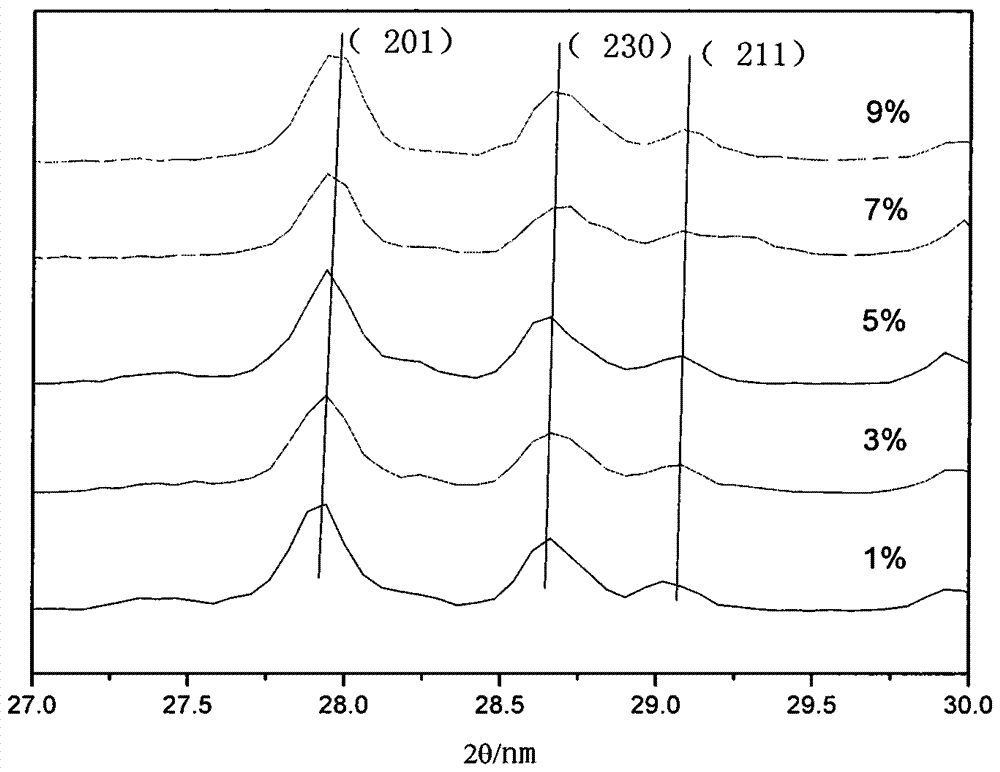
[0036] Pure La was analyzed using a DX2500 X-ray diffractometer 2 TiO 5 and different Dy 3+ Doped ratio samples were tested with a scan rate of 0.04° / min and a...
Embodiment 3
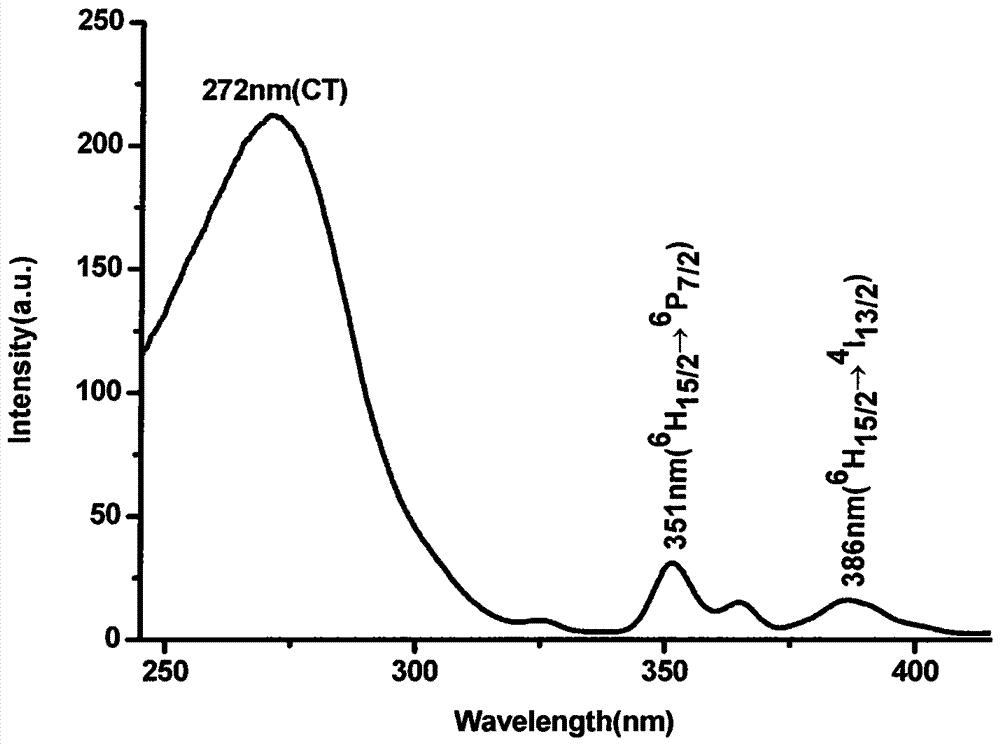
[0038] Weigh 1.98mmol lanthanum nitrate (La(NO 3 ) 3 , A.R.) was dissolved in an appropriate amount of distilled water, and 1 mmol of tetrabutyl titanate (C 16 h 36 o 4 Ti, A.R.), dissolved in 50 ml methanol. Weigh 0.02mmol dysprosium acetate (Dy(CH 3 COO) 3 , A.R.), dropwise added 10mmol nitric acid (HNO 3 , A.R.), stirred for 1.5h, and put into a water bath at 60°C for about 6h to obtain a colloidal precipitate, the precursor sol C. The precursor sol C was taken out and placed in a corundum crucible, calcined in a muffle furnace at 1100° C. for 4 hours, and kept for 1 hour to obtain the desired phosphor. image 3 In order to monitor the excitation spectrum at a wavelength of 577nm.
[0039] Such as Figure 4 As shown, a very strong charge transfer transition band (CT) appears in the range of 250-300nm, indicating that Dy 3+ Strong interaction with coordinated oxygen atoms. In addition, there are two very weak f-f electronic transition excitation peaks in the range...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com