Recombinant bacteria producing γ-aminobutyric acid and its construction method and application
A technology of aminobutyric acid and recombinant bacteria, applied in the direction of microorganism-based methods, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problem of low yield of bacterial cells and active proteins, difficult production process control, strong cytotoxicity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1, the construction of producing γ-aminobutyric acid genetically engineered bacteria
[0092] One, construct the recombinant plasmid expressing the coding gene of glutamic acid decarboxylase B
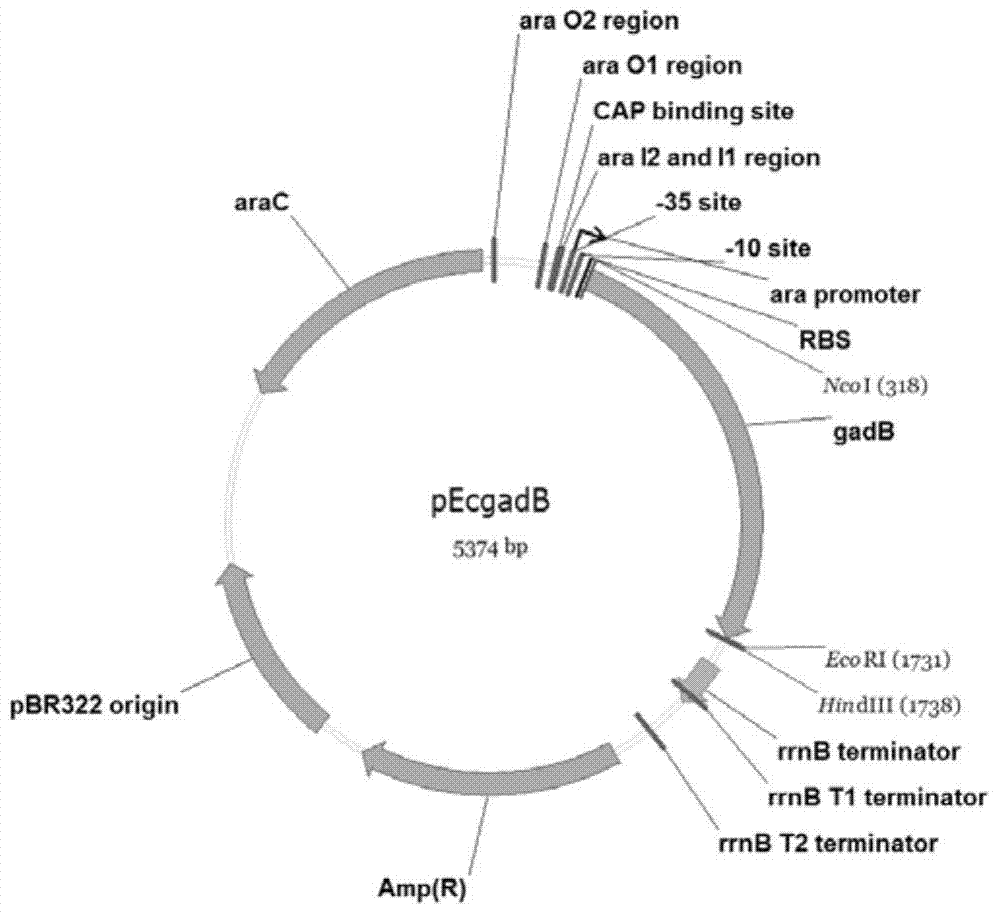
[0093] Replace the DNA sequence between the NcoI and EcoRI recognition sites of the pBAD / HisB vector with the DNA sequence used to encode glutamic acid decarboxylase B shown in SEQ ID No.4, keep other DNA sequences unchanged, and obtain the recombinant vector pEcgadB ( figure 1 ). Restriction identification proved that the gene encoding glutamic acid decarboxylase B was successfully inserted between the NcoI and EcoRI recognition sites of the pBAD / HisB vector. The recombinant vector pEcgadB can express glutamic acid decarboxylase B shown in SEQ ID No.1, and the nucleotide sequence shown in SEQ ID No.4 is the coding sequence of glutamic acid decarboxylase B.
[0094] 2. Construction of Escherichia coli mutant strain K12ΔgadAB with knockout γ-aminobutyric acid catabo...
Embodiment 2
[0114] Embodiment 2, producing gamma-aminobutyric acid genetically engineered bacteria transforming L-glutamic acid to produce gamma-aminobutyric acid
[0115] 1. Induction culture of genetically engineered bacteria producing γ-aminobutyric acid
[0116] The genetically engineered bacteria KG01, KG02, KG03, KG04, KG05, Escherichia coli K12ΔgabT (referred to as K12ΔgabT strain) and Escherichia coli K12 (referred to as K12 strain) were streaked to agar containing 1.5% mass concentration respectively. and ampicillin with a mass concentration of 100 μg / mL on LB plates, and cultured at 37°C for 12 hours. Pick a single colony on the plate, inoculate it into the liquid LB medium containing ampicillin with a mass concentration of 100 μg / mL, culture it overnight at 37° C. with shaking at 220 rpm; The inoculation amount was inoculated into the self-inducing medium ZYM, and the shaking culture was carried out at 200 rpm and 30°C for 16 hours to obtain the induced KG01 strain, KG02 strai...
Embodiment 3
[0135] Example 3 Preparation of γ-aminobutyric acid by genetically engineered bacteria KG01
[0136] Pick a single bacterium colony of the genetically engineered bacterium KG01 and inoculate it into the liquid LB medium containing ampicillin with a mass concentration of 100 μg / mL, cultivate overnight at 37° C. with shaking at 220 rpm; inoculate the overnight culture with 1% (volume percentage) Inoculate a large amount into a 100L fermenter equipped with 70L self-inducing medium ZYM, with an aeration ratio of 0.6-0.8vvm, and ferment for 16 hours at 30°C and a rotation speed of 300rpm to obtain a fermentation broth. Then use a tube centrifuge to centrifuge the fermentation broth, collect KG01 cells into a 150L transformation tank, add about 60L of pure water to resuspend the strains, and obtain the resuspended strains, so that the content of the cells in the resuspended strains is calculated by the wet weight of the cells It was calculated as 20g / L, and glutamic acid was added t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com