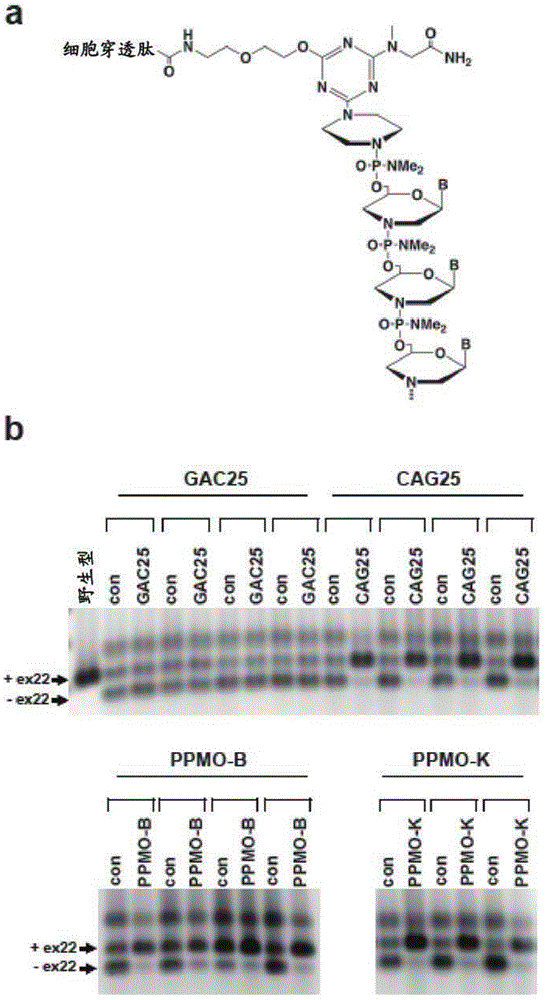
Peptide-linked morpholino antisense oligonucleotides for treatment of myotonic dystrophy
一种反义寡核苷酸、肌强直性的技术,应用在用于肌强直性营养不良治疗的肽连接吗啉代反义寡核苷酸领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In preparation of the formulations, it may be necessary to mill the active compound to provide the appropriate particle size prior to combining with the other ingredients. If the active compound is substantially insoluble, it is usually ground to a particle size of less than 200 mesh. If the active compound is substantially water soluble, the particle size is usually adjusted by milling to provide a substantially homogeneous distribution in the formulation, eg about 40 mesh.
[0055] Some examples of suitable excipients or carriers include lactose, glucose, sucrose, sorbitol, mannitol, starch, acacia, calcium phosphate, alginate, tragacanth, gelatin, calcium silicate, microcrystalline cellulose , Polyvinylpyrrolidone, Cellulose, Sterile Water, Syrup and Methylcellulose. The formulations may additionally include: lubricating agents such as talc, magnesium stearate, and mineral oil; wetting agents; emulsifying and suspending agents; preservatives such as methyl- and prop...
Embodiment 1
[0114] Example 1: Intramuscular injection of two different PPMO conjugates into the tibialis anterior muscle to correct abnormal RNA splicing
[0115] The large expansion of CUG trinucleotide sequences provides the basis for the gain-of-function of toxic RNAs leading to detrimental changes in RNA metabolism. Such a CUG repeat element is normally located in the 3' untranslated region of the protein kinase DMPK, but when expanded it becomes the genetic lesion of myotonic dystrophy type I (DM1), an inherited neuromuscular disease. Pathogenic DMPK transcripts containing CUG expansions are retained in ribonuclear foci as part of complexes with RNA-binding proteins such as muscle blindness-like 1 (MBNL1), leading to aberrant splicing of numerous RNA transcripts and physiological abnormalities including myotonia.
[0116] In this example, a condensation synthesis reaction was performed to generate CAG25 comprising a morpholine based on the 25 mg mer CAG sequence (Wheeler et al., ...
Embodiment 2
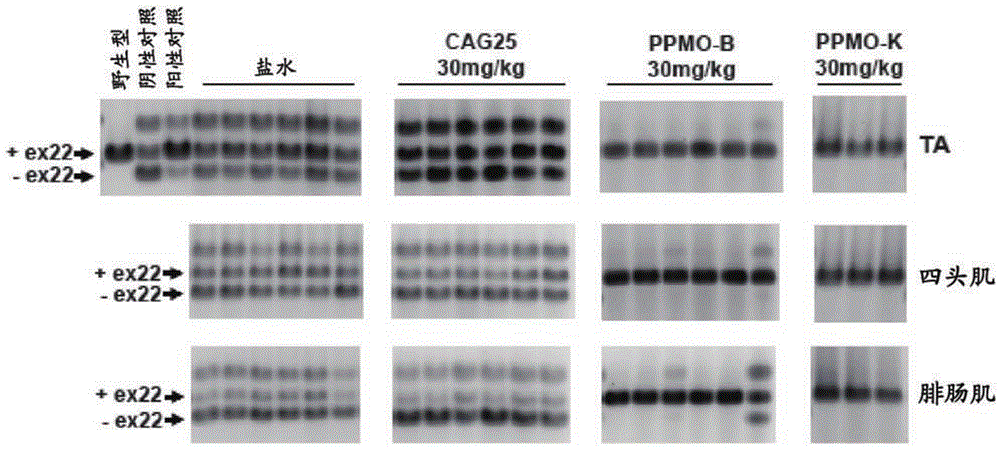
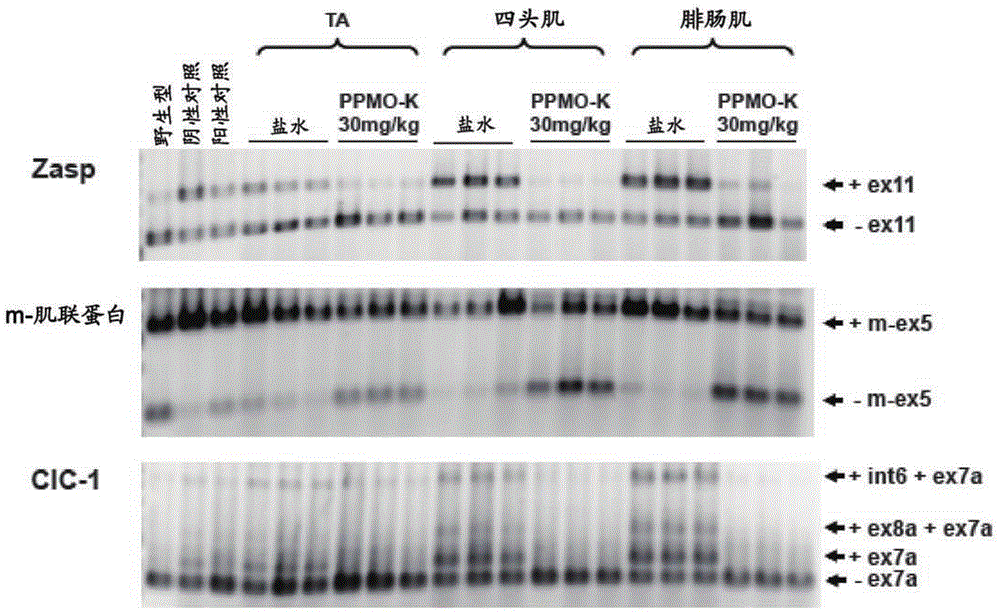
[0130] Example 2: Repeated IV injections of PPMO-B and PPMO-K are effective in ameliorating splicing diseases in vivo
[0131] Having confirmed that modification of CAG25 with peptides B or K does not abolish its biological activity, this example evaluates whether PPMO-B and PPMO-K can modulate HSA using an IV dosing regimen LR Effects of toxic RNA in mice.
[0132] Materials and methods
[0133] HSA LR Systematic Delivery Studies in
[0134] CAG25, PPMO-B and PPMO-K were dissolved in saline and administered to male and female HSA via tail vein IV injection at a dose of 30 mg / kg body weight LR Homozygous mice, once a week for six weeks. Blood was collected by orbital bleeding 24 hours after the last dose. Approximately one week after the final dose, mice were assessed for the presence of myotonia as described in the electromyography procedure. Mice were subsequently sacrificed and muscle sections were either frozen in liquid nitrogen for RNA analysis or embedded ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com