A kind of insulin liposome penetration enhancer and preparation method thereof
A technology of transdermal enhancer and liposome, which is applied in liposome delivery, pharmaceutical formulations, peptide/protein components, etc., can solve problems such as the complexity of the triggering mechanism, increase the transdermal rate, and promote liposome penetration. skin, no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Insulin liposome transdermal enhancer and synthesis of insulin liposome.
[0028] (1) Accurately weigh 1 g of kojic acid, dissolve it in 100 g of Phosphate Buffered Saline-Tween-20 (PBS-T), shake it for 5 minutes to obtain the accelerator, and then put it in a 4-degree refrigerator Save it for later use and take it out when in use.
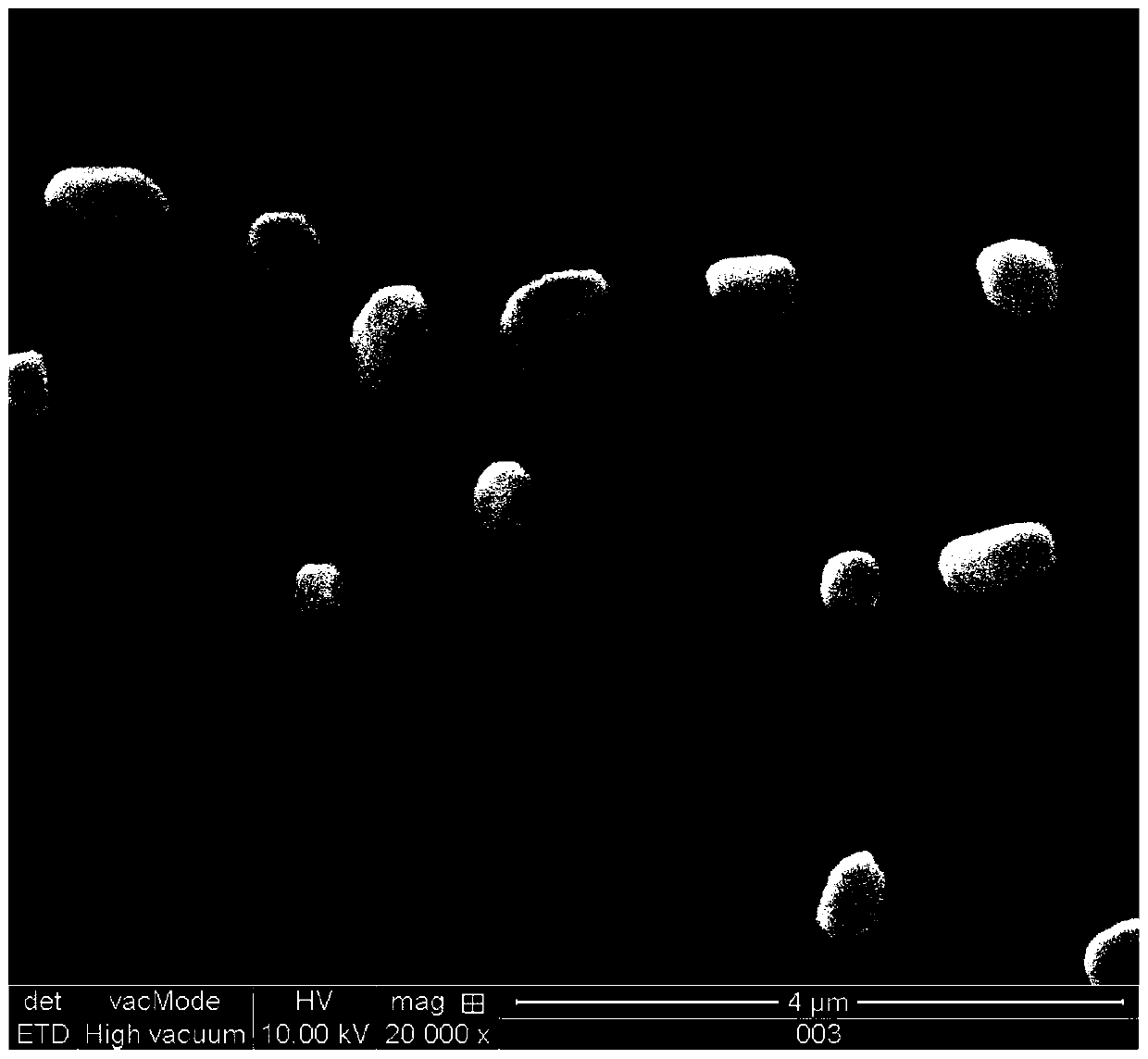
[0029] (2) Preparation of insulin liposome: the rotary evaporation method prepares soybean lecithin film (lecithin 25mg, cholesterol 5mg), controls temperature during rotary evaporation to be 45 ℃, then will contain the triethanolamine hydrochloride buffer solution (1mg) of insulin ( 15mL) was poured into the film; the probe-type ultrasonic instrument was used for 10 minutes, and then placed on a rotary evaporator to rotate for 1 hour to obtain the corresponding liposome gel solution. Wherein, step (2) is embodiment 1a.
[0030] (3) Preparation of insulin flexible liposomes: prepare soybean lecithin film (lecithin 25mg, cholesterol 5mg) b...
Embodiment 2
[0034] Rat skin collection and in vitro transdermal experiment.
[0035] (1) Randomly select 10 SD rats with a body weight of 130-150g. The rats were bought and fed adaptively for 2 days before they were killed. The rat skin was peeled off, and after the abdominal skin was specially collected, the superficial adipose tissue was removed. As for storage at -80°C refrigerator.
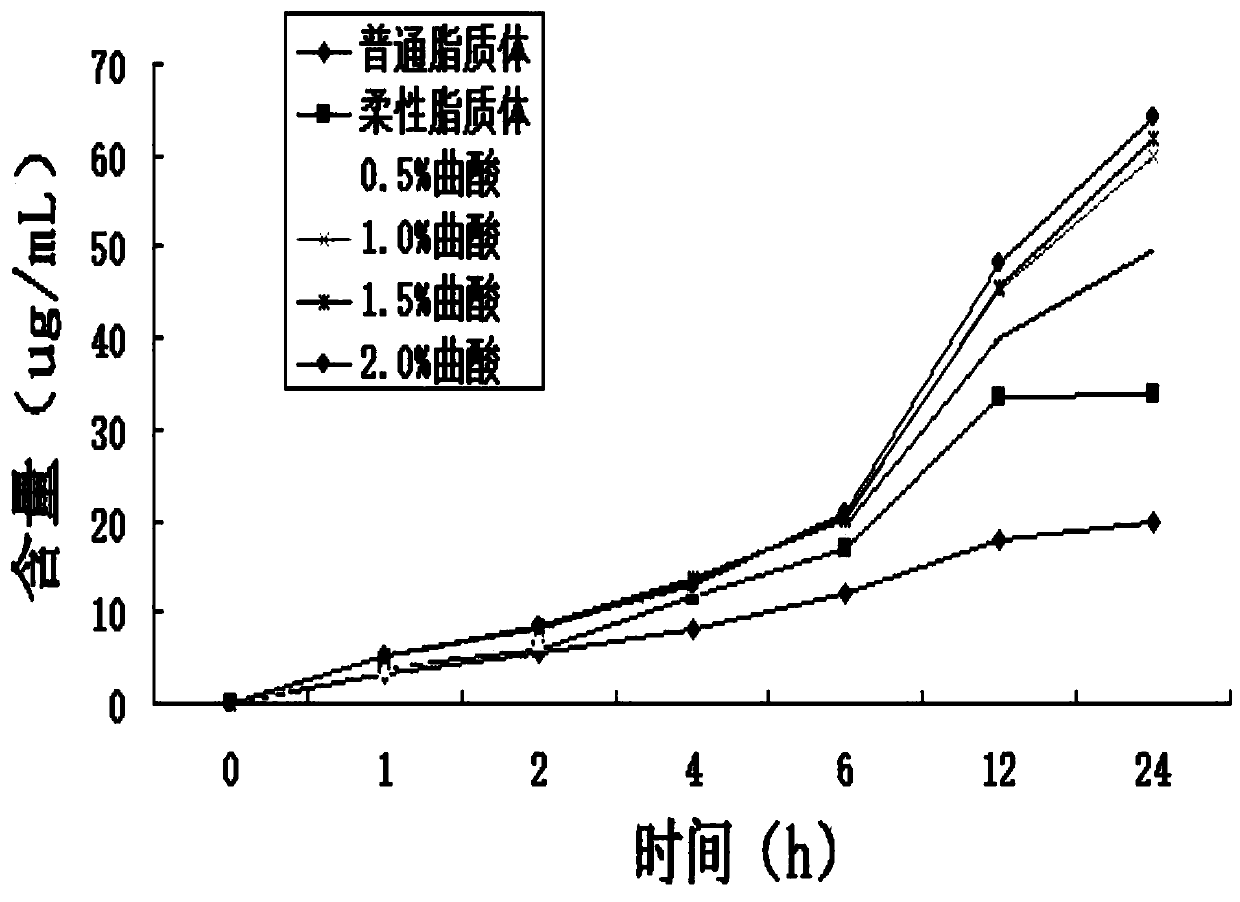
[0036] (2) Get the skin of the rat and put it on the transdermal instrument, give different groups of corresponding kojic acid solutions (mass fraction is 0.5%, 1.0%, 1.5% and 2.0%) to process, give insulin liposome 500ug (embodiment 1a Insulin liposome) in Example 1a) was applied, and the ordinary liposome in Example 1a was applied to rat skin without kojic acid treatment, and the flexible liposome in Example 1b was applied to rat skin without kojic acid treatment. Rat skin, test rat skin transdermal amount, and compare with ordinary liposome and flexible liposome, as shown in Figure 2. Wherein, the pe...
Embodiment 3
[0038] In vivo hypoglycemic observation of insulin liposomes.
[0039] (1) Establishment of type 2 diabetes rat animal model, give high-fat feed to feed for 6 months to establish type 2 diabetes rat model, high-fat feed consists of 10% lard, 18.9% protein powder, 70% common feed , 1% cholesterol and 0.1% sodium cholate.
[0040] (2) At the beginning of the experiment, the hair on the surface of the rat was pushed away, and after kojic acid treatment of different concentrations (mass fraction was 0.5%, 1.0%, 1.5% and 2.0%), the insulin liposome (in Example 1a) was coated. Insulin liposome), in addition, choose the common liposome in Example 1a and apply it to the rat skin without kojic acid treatment, and get the flexible liposome in Example 1b and apply it to the rat skin without kojic acid treatment At 0h, 1h, 2h, 3h, 4h, 5h, and 6h, blood was taken from the tail vein, and blood glucose was measured with a Sinocare blood glucose meter to observe the hypoglycemic characterist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com