Copper-based Cu-Cu2O-CuO catalyst as well as preparation method and application thereof
A cu-cu2o-cuo, catalyst technology, applied in the field of chemical catalysts, can solve the problems of uneven mixing between components, catalyst gap, high catalyst production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Catalyst preparation:
[0077] (1) Weigh 37.5g copper sulfate pentahydrate and add it to 300mL deionized water to form a copper salt solution (copper ion concentration is 0.5mol / L);
[0078] (2) Add 50mL 6mol / L NaOH solution to the resulting solution and stir to obtain Cu(OH) 2 Precipitate, control and stir at 75°C for 10min so that Cu(OH) 2 Precipitation is transformed into CuO precipitation;
[0079] (3) Add 2.52 mL of 80% N 2 h 4 ·H2 O solution was stirred at 70°C for 1.5h. After the reaction, the obtained reaction product was filtered, washed with water and absolute ethanol for 5 times, and vacuum-dried at 60°C for 8h to obtain a controllable multi-component copper-based catalyst powder.
[0080] The composition (mass percentage) of gained copper catalyst after chemical analysis is: Cu 14.5%, Cu 2 O 70.1% and CuO 15.4%.
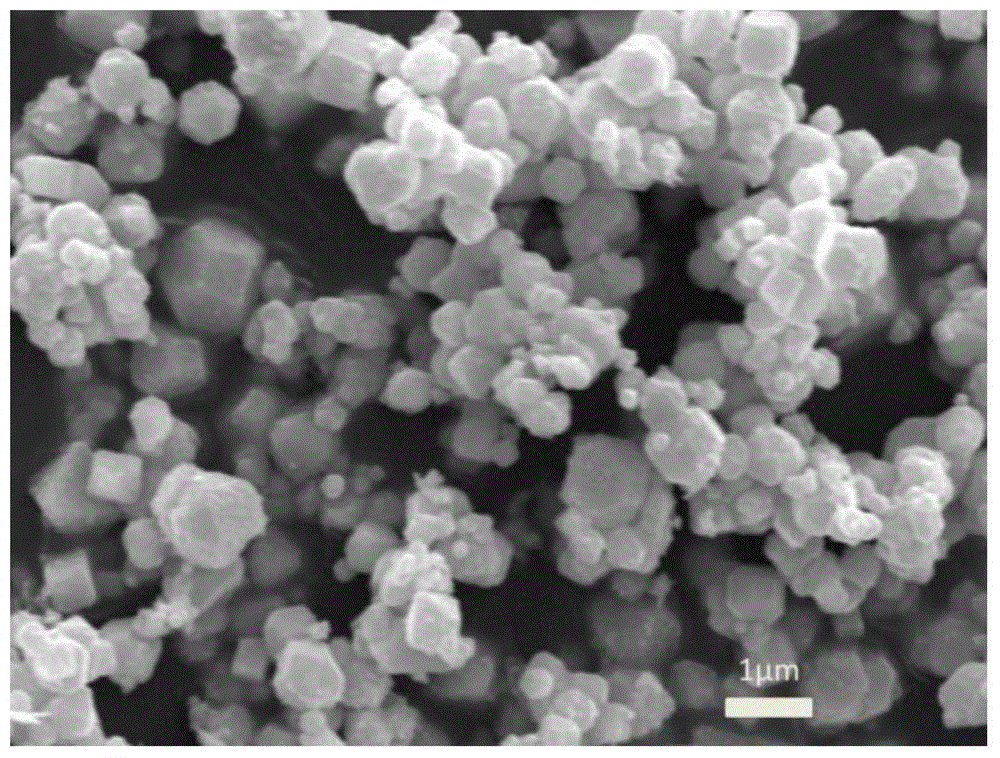
[0081] Catalyst Characterization:
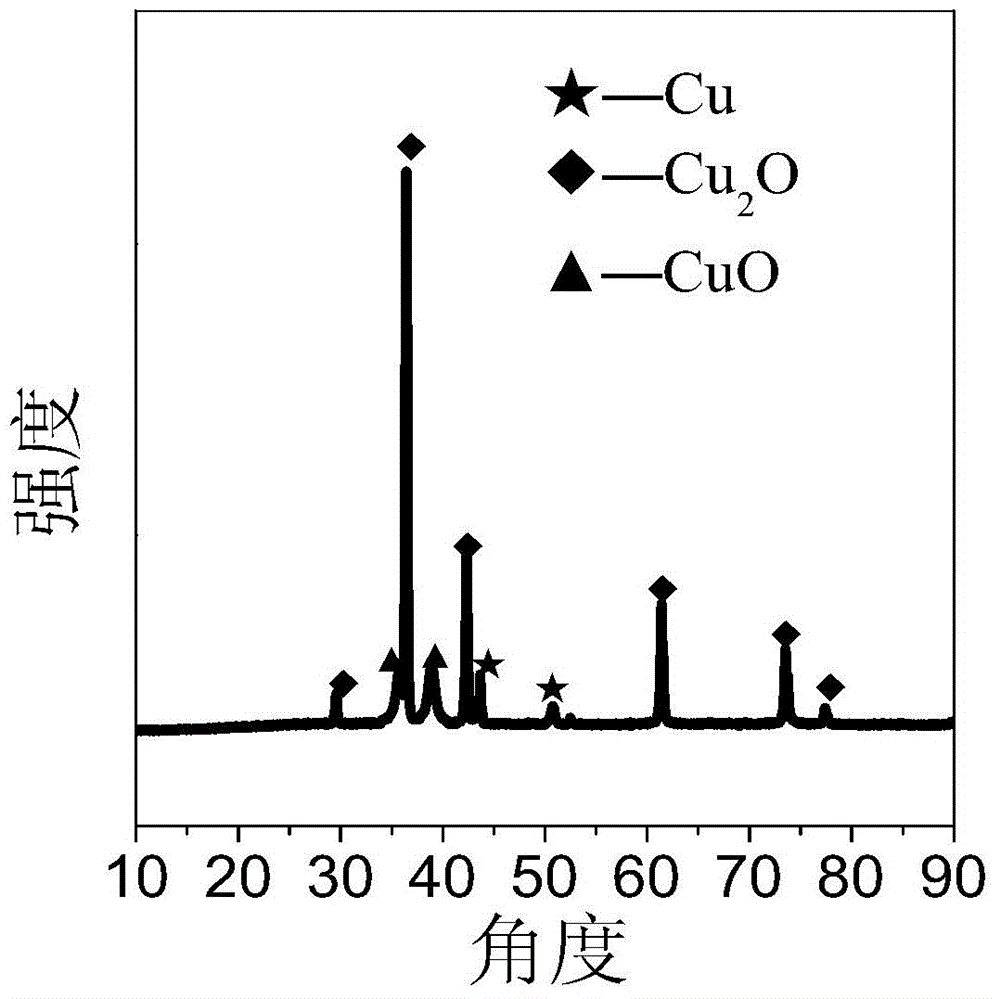
[0082] figure 1 It is the XRD figure of the copper catalyst that embodiment 1 obtains, wherein 2θ=36.4...
Embodiment 2
[0086] Catalyst preparation:
[0087] (1) Take 25g of copper sulfate pentahydrate and add it to 400mL alcohol-water mixed solution (wherein the volume percentage of ethanol is 10%, and the volume percentage of water is 90%) to form a copper salt solution (copper ion concentration is 0.25mol / L) ;
[0088] (2) Adding 50mL of NaOH solution with a concentration of 4mol / L in the resulting solution stirred to obtain Cu(OH) 2 Precipitate, control and stir at 72°C for 20min so that Cu(OH) 2 Precipitation is transformed into CuO precipitation;
[0089] (3) Add 2.21mL of 80% N 2 h 4 ·H 2 O solution, stirred at 80° C. for 1 h, filtered the obtained reaction product after the reaction, washed 5 times with water and absolute ethanol, and vacuum-dried at 70° C. for 8 h to obtain a controllable multi-component copper-based catalyst powder.
[0090] Gained copper catalyst composition (mass percentage) is as follows after chemical method analysis: Cu 18.2%, Cu 2 O 68.2% and CuO 13.6%. ...
Embodiment 3
[0096] Catalyst preparation:
[0097] (1) Weigh 37.5g copper sulfate pentahydrate and add it to 300mL deionized water to form a copper salt solution (copper ion concentration is 0.5mol / L);
[0098] (2) Add 50mL 6mol / L NaOH solution to the resulting solution and stir to obtain Cu(OH) 2 Precipitate, control and stir at 75°C for 10min so that Cu(OH) 2 Precipitation is transformed into CuO precipitation;
[0099] (3) Add dropwise 1.56mL of 80% N 2 h 4 ·H 2 O solution, stirred at 70°C for 2h, after the reaction, the obtained reaction product was filtered, washed with water and absolute ethanol for 5 times, and vacuum-dried at 60°C for 8h to obtain a controllable multi-element copper-based catalyst powder.
[0100] The composition (mass percentage) of the obtained copper catalyst after chemical analysis is as follows: Cu2O 70.1% and CuO 29.9%.
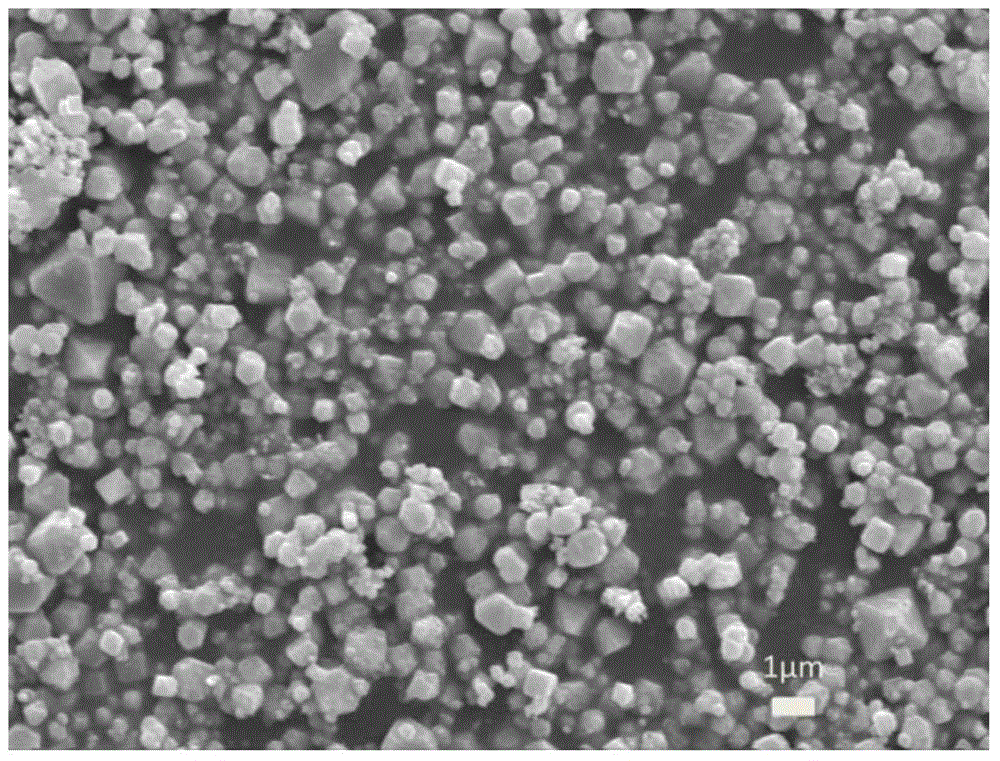
[0101] Catalyst Characterization:
[0102] Figure 7 CuO-Cu obtained for embodiment 3 2 XRD pattern of O catalyst, where 2θ=36.4° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com