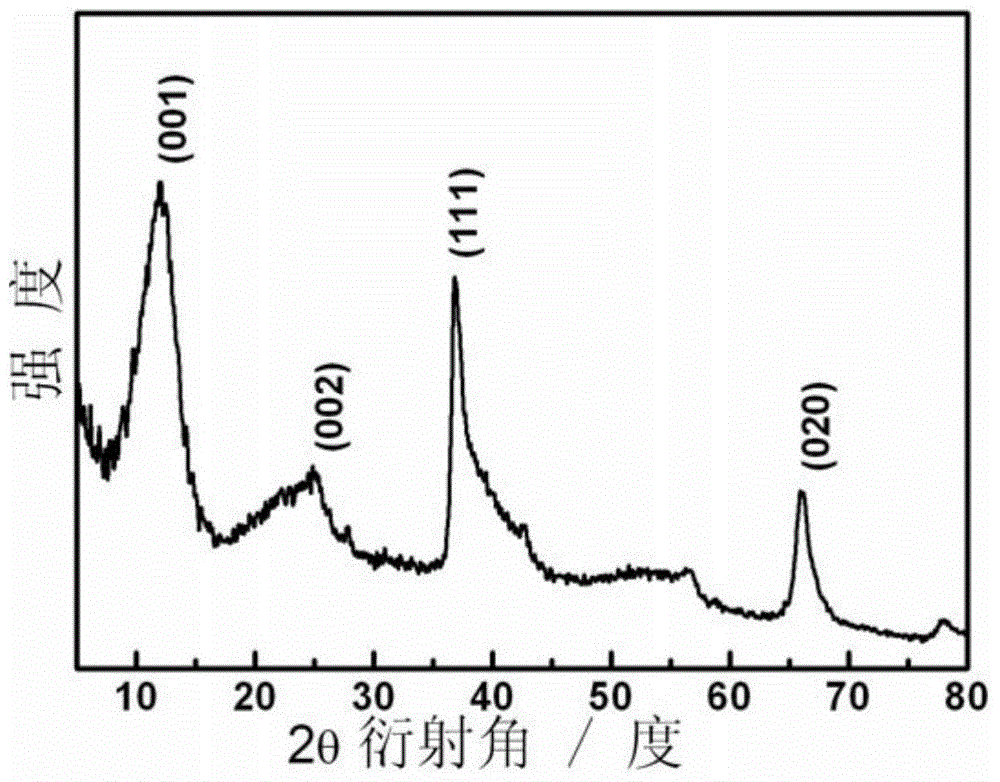
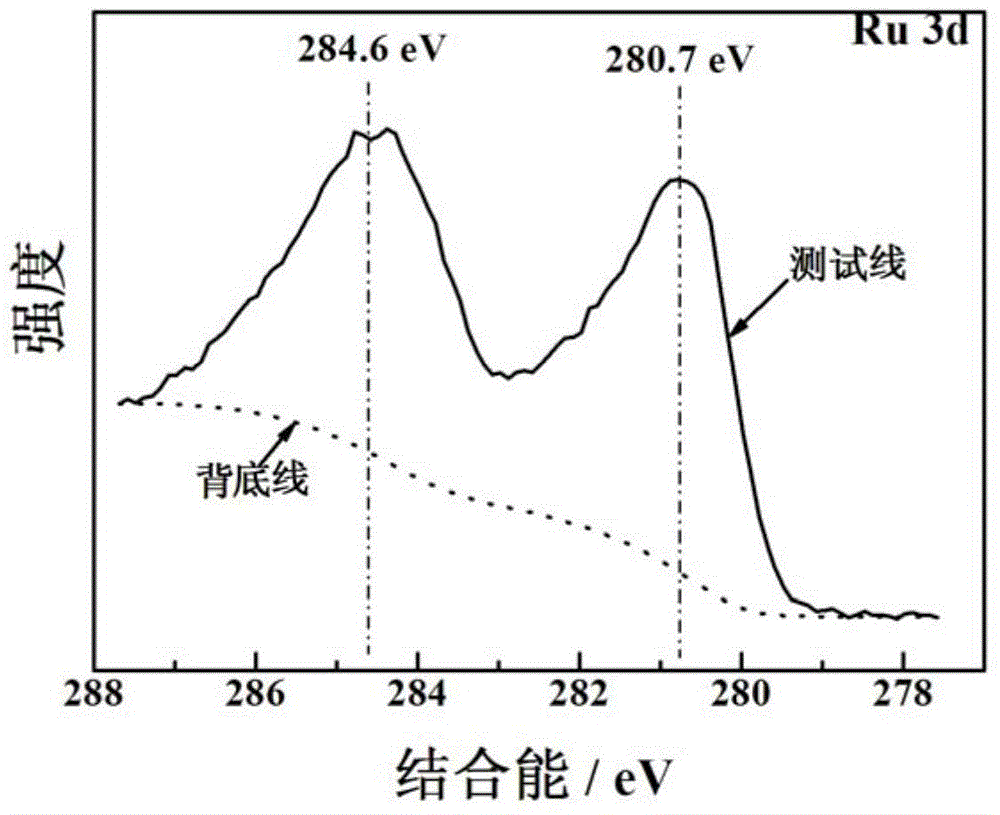
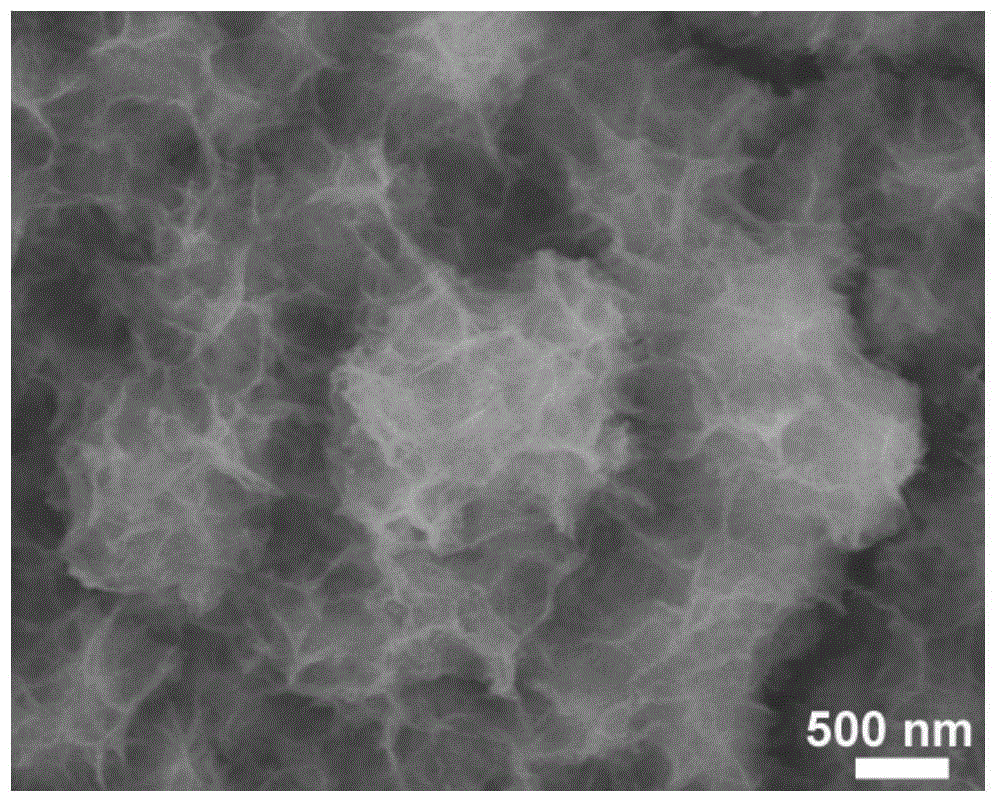
Porous ruthenium dioxide and manganese dioxide combined electrode and preparation method and application thereof
A composite electrode and porous technology is applied in the field of porous RuO2/MnO2 composite electrode and its preparation, and achieves the effects of easy decomposition, improved electrochemical performance and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] KMnO 4 and 96 wt% H 2 SO 4 (molar mass KMnO 4 0.01) was dissolved in deionized water, stirred evenly, prepared with K + A solution with a concentration of 0.01mol / L. Use nickel foam as a matrix, immerse in the above solution, then transfer it into a reaction kettle, seal it and keep it in an oven at 85°C for 1 hour, then rinse it with deionized water and absolute alcohol several times, and dry it in a vacuum oven at 60°C for 12 Hours later, the manganese-containing precursor supported on Ni was obtained; the obtained manganese-containing precursor supported on Ni was calcined at 300 ° C for 2 hours in an Ar atmosphere, and then cooled to room temperature to obtain MnO2 supported on nickel foam. 2 Electrode (Ni / MnO 2 ), where MnO 2 The loading capacity is 0.3mg / cm 2 ; The above-mentioned MnO carried on the nickel foam 2 The electrode is immersed in RuCl 3 (Concentration: 4mg / mL), soaked for 3 hours, rinsed with deionized water and absolute ethanol several times,...
Embodiment 2
[0059] KMnO 4 and 96 wt% H 2 SO 4 (molar mass KMnO 4 0.04) was dissolved in deionized water, stirred evenly, prepared with K + A solution with a concentration of 0.02mol / L. Use nickel foam as a matrix, immerse in the above solution, and then transfer it into a reaction kettle, seal it and keep it in an oven at 70°C for 1.5 hours, then rinse it with deionized water and absolute alcohol several times, and dry it in a vacuum oven at 60°C for 12 hours. Hours later, the manganese-containing precursor supported on Ni was obtained; the obtained manganese-containing precursor supported on Ni was calcined at 400 ° C for 1 hour in an Ar atmosphere, and then cooled to room temperature to obtain MnO supported on nickel foam. 2 Electrode (Ni / MnO 2 ), where MnO 2 The loading capacity is 0.4mg / cm 2 ; The above-mentioned MnO carried on the nickel foam 2 The electrode is immersed in RuCl 3 (Concentration: 4 mg / mL), soaked for 5 hours, rinsed with deionized water and absolute ethanol s...
Embodiment 3
[0064] KMnO 4 and 96 wt% H 2 SO 4 (molar mass KMnO 4 0.02) was dissolved in deionized water, stirred evenly, prepared with K + A solution with a concentration of 0.01mol / L. Use foamed nickel as a matrix, immerse in the above solution, then transfer it into the reaction kettle, keep it in a 90°C oven for 0.5 hours after sealing it, then rinse it with deionized water and absolute alcohol several times, and vacuum dry it in a 60°C oven for 12 Hours later, the manganese-containing precursor supported on Ni was obtained; the obtained manganese-containing precursor supported on Ni was calcined at 300 ° C for 2.5 hours in an Ar atmosphere, and then cooled to room temperature to obtain MnO2 supported on nickel foam. 2 Electrode (Ni / MnO 2 ), where MnO 2 The loading capacity is 0.25mg / cm 2 ; The above-mentioned MnO carried on the nickel foam 2 The electrode is immersed in RuCl 3 (Concentration: 3 mg / mL), soaked for 1.5 hours, rinsed with deionized water and absolute ethanol sev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com