Preparation method of manganese sulfate monohydrate
A technology for hydrating sulfuric acid and manganese sulfate, applied in the field of chemistry, can solve problems such as difficulty and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
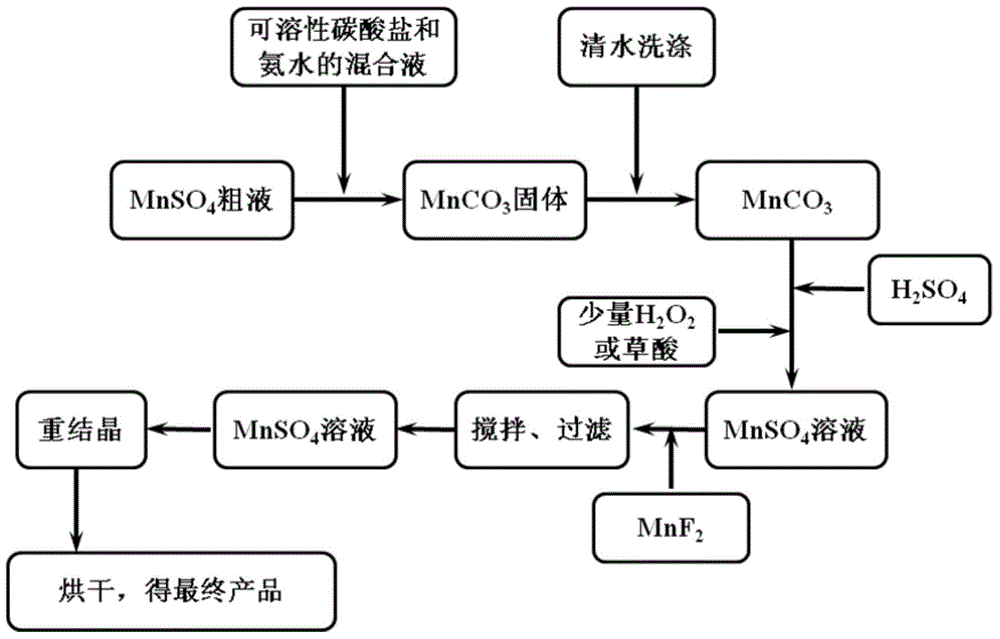
[0035] Get the manganese sulfate solution that rhodochrosite is made, measure the Mg content wherein with inductively coupled plasma emission spectrometer to be 8473mg L -1 , Ca content 2150mg·L -1 , K content 1073mg·L -1 , Na content 1235mg·L -1 , Fe content 151mg·L -1 , Ni content 26mg·L -1 , Cu content 37mg·L -1 , Zn content 63mg·L -1 , Pb content 50mg·L -1 . Simultaneous determination of Mn 2+ The content is 42.5g·L -1 .
[0036] Accurately measure 1000mL of manganese sulfate solution and place it in a 2000mL volumetric flask, stir and heat to 60°C, then slowly add 64.13g of NaCO 3 and 50 mL of ammonia water (25% to 28%), and stirred at this temperature for 60 min. After separation by filtration, the solid was mixed at a material-to-water ratio of 1:5 and stirred and washed at 60°C for 60 minutes. After filtration, the filter cake was beaten with a small amount of water, and then 12mol / L sulfuric acid was slowly added to it, and the amount of sulfuric acid was ...
Embodiment 2
[0038] Accurately measure 1000mL of manganese sulfate solution and place it in a 2000mL volumetric flask, stir and heat to 60°C, then slowly add 61.1g of NH 4 HCO 3 and 68mL of ammonia water (25wt%-28wt%), stirred at this temperature for 60min. After separation by filtration, the solid was mixed at a material-to-water ratio of 1:5 and stirred and washed at 50°C for 60 minutes. After filtration, the filter cake was beaten with a small amount of water, and 12mol / L sulfuric acid was slowly added to it, and the amount of sulfuric acid was controlled so that the pH was 4, and then 1mL hydrogen peroxide was added to it, stirred at 50°C for 60min, and then mixed with MnCO 3 After adjusting pH=5-6, filter. Add 0.75g MnF to the filtrate with stirring at 50°C 2 Stir and react for 120 minutes, filter, discard the filter cake, follow the recrystallization process of the filtrate, and dry the obtained solid at 80°C for 24 hours to obtain a high-purity manganese sulfate solid, which is r...
Embodiment 3
[0040] Accurately measure 1000mL of manganese sulfate solution and place it in a 2000mL volumetric flask, stir and heat to 80°C, then slowly add 72.7g of (NH 4 ) 2 CO 3 In a mixed solution formed with 75mL of ammonia water (25%-28%), stir at this temperature for 60min. After separation by filtration, the solid was mixed at a material-to-water ratio of 1:6 and stirred and washed at 80°C for 60 minutes. After filtration, the filter cake was beaten with a small amount of water, and 12mol / L sulfuric acid was slowly added to it, and the amount of sulfuric acid was controlled so that the pH was 4, and then 0.3g oxalic acid was added to it, stirred at 80°C for 60min, and then mixed with MnCO 3 After adjusting pH=5-6, filter. Add 0.3g MnF to the filtrate under stirring at 40°C 2 Stir and react for 120 minutes, filter, discard the filter cake, follow the recrystallization process of the filtrate, and dry the obtained solid at 80°C for 24 hours to obtain a high-purity manganese sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com