Propylene acyloxy methacryloxy silane and preparation method of its derivative
A technology of acryloyloxymethacryloyloxysilane and methacrylic acid, which is applied in the field of synthesis of organosilicon compounds, can solve the problems of expensive catalyst reaction conditions, low catalytic efficiency, and easy polymerization of double bonds, and achieve easy industrialization The effect of high production, high yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of acryloyloxymethacryloyloxydimethylsilane
[0039] The structural formula of acryloxymethacryloxydimethylsilane is as formula (IV):
[0040]
[0041] Including the following steps:
[0042] Put 14.79g of acrylic acid, 0.24g of p-tert-butyl catechol, 0.37g of tetrabutyl chlorinated Ammonium and 50.02g of anhydrous xylene, slowly add 14.17g of chloromethyl dimethyl chlorosilane dropwise into the reaction flask, the rate of dropping is 2ml / min, stir slowly, after the dropwise addition is completed, keep the reaction at 90°C After 8 hours, after the completion of the reaction, cool to room temperature naturally, filter under reduced pressure, remove toluene from the filtrate, and distill under reduced pressure at a pressure of 2 to 3 mmHg to obtain a colorless and transparent liquid product with a yield of 79.86%.
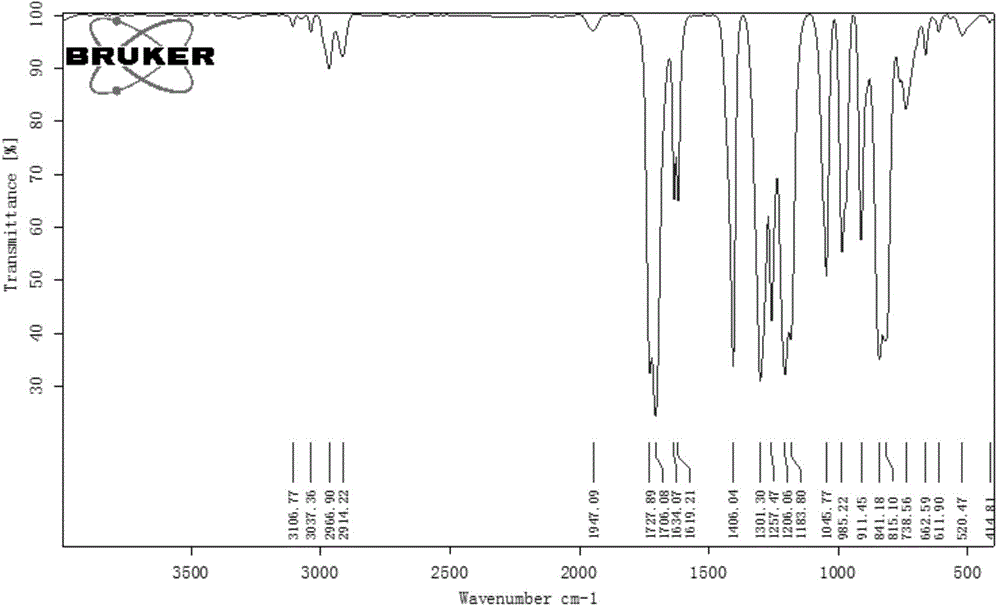
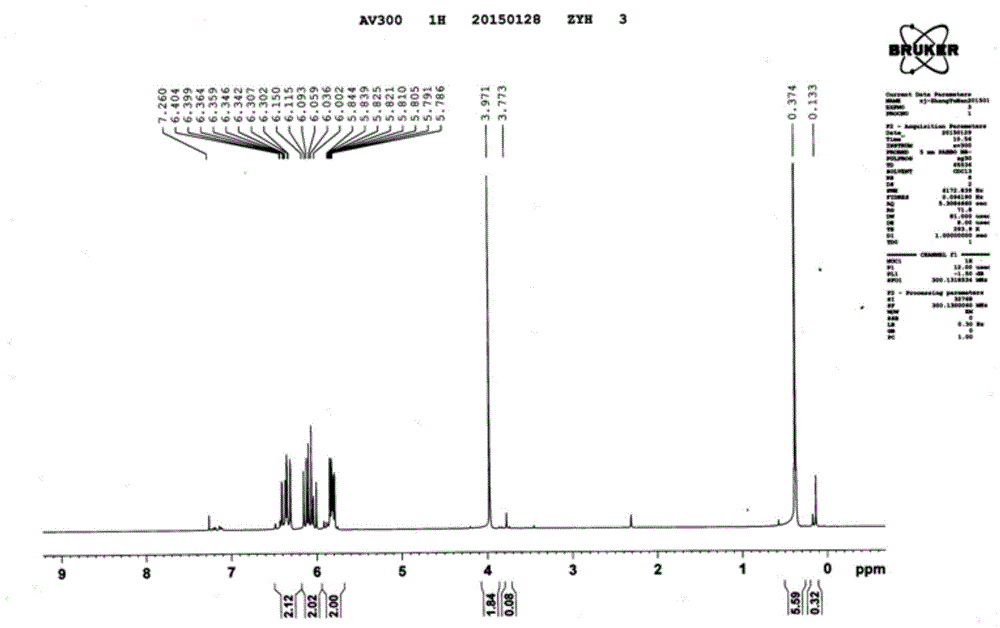
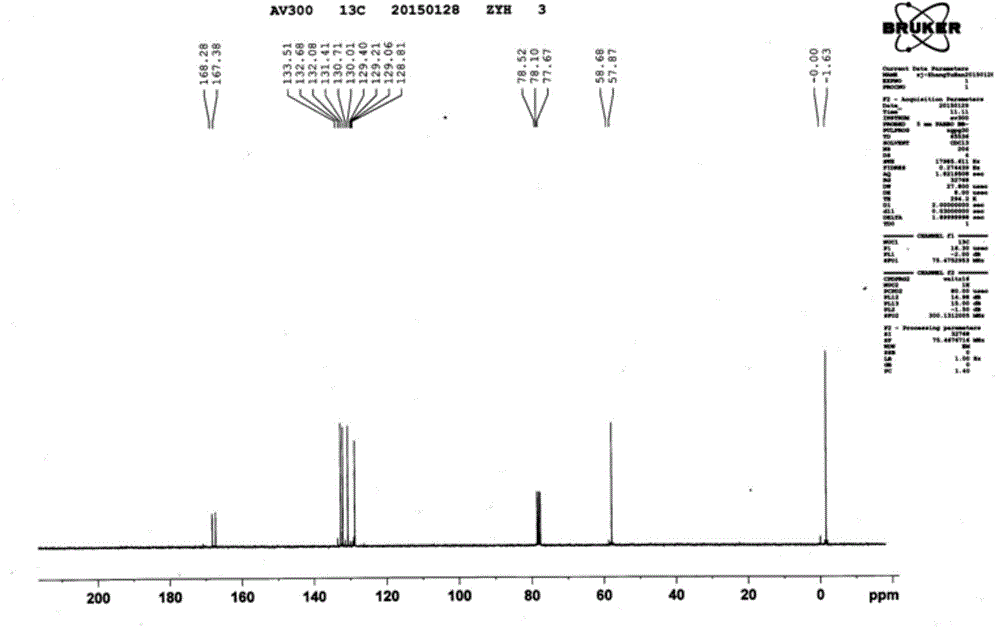
[0043] The infrared of the product obtained in this embodiment, the proton nuclear magnetic spectrum and the carbon spectru...
Embodiment 2
[0044] Embodiment 2, the preparation of acryloyloxymethacryloyloxydimethylsilane
[0045] The structural formula of acryloyloxymethacryloyloxydimethylsilane is as formula (IV),
[0046] Including the following steps:
[0047] Put 18.81g of sodium acrylate, 0.20g of p-tert-butylcatechol, 0.58g of tetrabutyl bromide into a four-necked round-bottomed flask equipped with a spherical condenser, a thermometer, a constant pressure dropping funnel, and a T-shaped three-way piston. Add phosphine and 50.28g of anhydrous toluene, and slowly add 14.21g of chloromethyl dimethyl chlorosilane into the reaction flask dropwise, the rate of addition is 2ml / min, stir slowly, after the addition is completed, keep the temperature at 85°C for reaction After 8 hours, after the completion of the reaction, cool to room temperature naturally, filter under reduced pressure, remove toluene from the filtrate, and distill under reduced pressure at a pressure of 2 to 3 mmHg to obtain a colorless and transp...
Embodiment 3
[0048] Embodiment 3, the preparation of acryloyloxymethacryloyloxymethylethylsilane
[0049] The structural formula of acryloyloxymethacryloyloxymethylethylsilane is as formula (Ⅴ):
[0050]
[0051] Including the following steps:
[0052] Put 22.03g of potassium acrylate, 0.36g of 4-4-methylenebis(2,6- Di-tert-butylphenol), 0.71g tetrabutylphosphine bromide and 52.36g anhydrous toluene, 15.70g chloromethylmethyl ethyl chlorosilane is slowly added dropwise in the reaction flask, and the rate of addition is 1.5ml / min, stir slowly, after the dropwise addition is completed, keep the temperature at 100°C for 12 hours, after the reaction is completed, naturally cool to room temperature, filter under reduced pressure, remove toluene from the filtrate, distill under reduced pressure, the pressure is 2 ~ 3mmHg, and the colorless and transparent Liquid product, yield 78.17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com