Aromatic amine schiff base derivative of gossypol and preparation method and plant-virus resisting application thereof
A technology of aromatic amine Schiff base and anti-plant virus agent, which is applied in the field of pesticides, can solve the problems of reducing the severity of symptoms, not many, etc., and achieve the effects of low toxicity, easy preparation, and significant anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
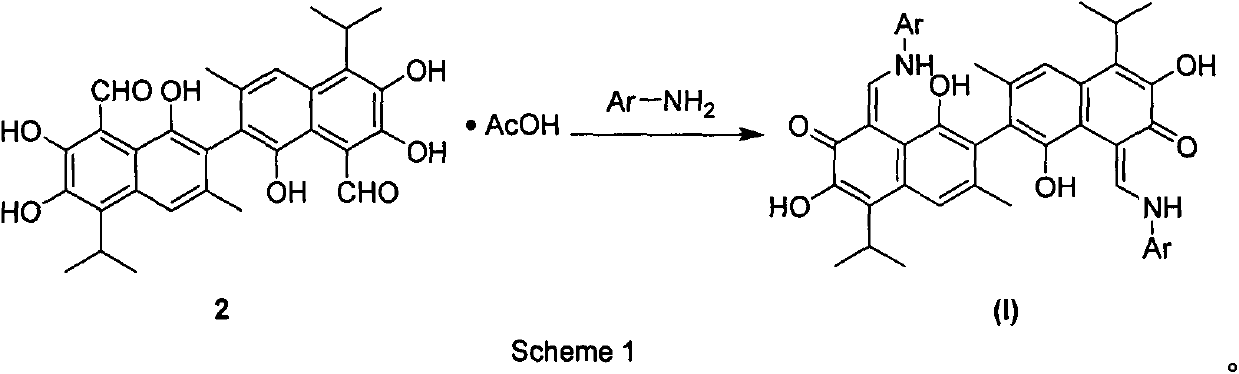
[0022] Embodiment 1: the synthesis of gossypol-aniline Schiff base (3):
[0023]
[0024] In a 100mL round bottom bottle, add 0.50g (0.86mmol) of gossypol acetate and 40mL of absolute ethanol, and then add 0.16g (1.73mmol) of aniline after fully dissolving. The reaction solution was heated to reflux for 5 hours. After naturally cooling to room temperature, the reaction solution was filtered, and the solid was recrystallized from benzene, then suction filtered and dried to obtain the target compound. Yield, 95%; melting point: 252-253°C; 1 H NMR (400MHz, CDCl 3 )δ10.17(d, J=12.3Hz, 2H), 7.89(s, 2H), 7.64(s, 2H), 7.37(t, J=7.5Hz, 4H), 7.30(d, J=7.9Hz, 2H), 7.19(t, J=6.9Hz, 2H), 5.77(s, 2H), 3.70-3.77(m, 2H), 2.16(s, 6H), 1.51-1.57(m, 12H); HRMS[ESI ]calcd for C 42 h 39 N 2 o 6 (M-H)-667.2814; found, 667.2812.
Embodiment 2
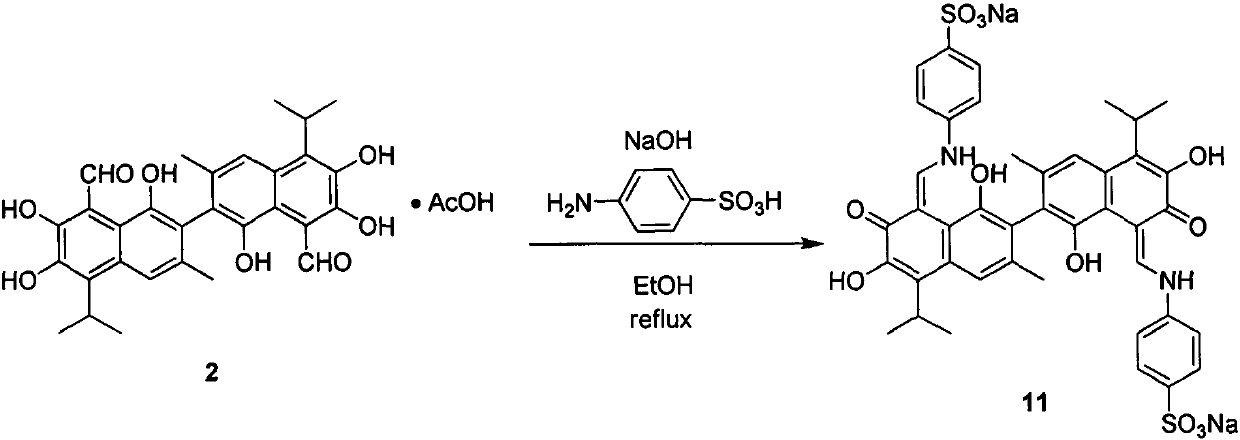
[0025] Embodiment 2: the synthesis of gossypol-4-sodium sulfonic acid aniline Schiff base (11)
[0026]
[0027] In a 100mL round bottom bottle, add 0.11g (2.59mmol) of sodium hydroxide, 40mL of absolute ethanol, and 0.30g (1.73mmol) of p-aminobenzenesulfonic acid, heat and reflux for 1 hour to generate sodium salt, then add 0.50mL of gossypol acetate g (0.86 mmol), continued heating for 5 hours, cooled to room temperature, filtered with suction, and recrystallized with isopropanol and methanol to obtain 0.27 g of a purple solid. Yield: 36%; Melting point >300°C; 1 H NMR (400MHz, DMSO-d 6 )δ14.94(d, J=11.7Hz, 2H), 10.41(d, J=11.7Hz, 2H), 8.58(s, 2H), 8.34(s, 2H), 7.64(d, J=8.0Hz, 4H), 7.52(s, 2H), 7.29(d, J=8.0Hz, 4H), 3.80-3.70(m, 2H), 2.00(s, 6H), 1.52-1.43(m, 12H).HRMS[ESI ]calcd for C 42 h 39 N 2 o 12 S 2 (M-2Na+H) - 827.1950, found 827.1928.
Embodiment 3
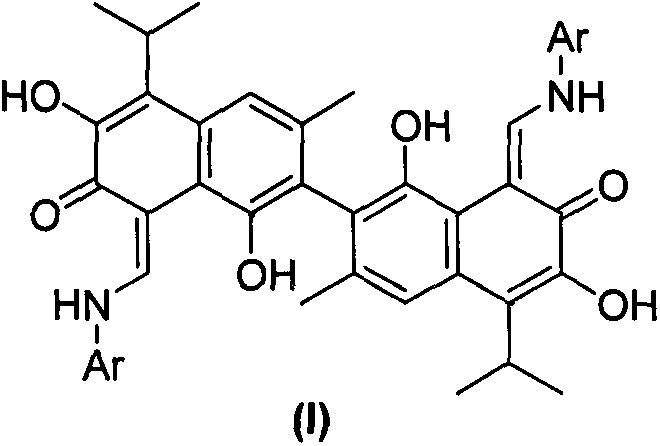
[0028] Embodiment 3: the synthesis of aromatic amine Schiff base derivatives 4-10, 12-22, 24-25 of gossypol: complete by repeating the method of Example 1
[0029] Synthesis of gossypol-4-methylaniline Schiff base (4):
[0030] Orange solid. Yield: 95%; Melting point: 246-248°C; 1 H NMR (400MHz, CDCl 3 )δ10.14(d, J=12.3Hz, 2H), 7.91(s, 2H), 7.63(s, 2H), 7.20(d, J=8.35Hz, 4H), 7.15(d, J=8.35Hz, 4H), 5.75(s, 2H), 3.71-3.77(m, 2H), 2.33(s, 6H), 2.15(s, 6H), 1.55(d, J=6.0Hz, 12H); HRMS[ESI]calcdfor C 44 h 43 N 2 o 6 (M-H) - , 695.3127; found, 695.3139.
[0031] Synthesis of gossypol-4-methoxyaniline Schiff base (5):
[0032] Dark yellow solid. Yield, 60%; Melting point: 272-274°C; 1 H NMR (400MHz, CDCl 3 )δ15.08(d, J=12.0Hz, 2H), 10.09(d, J=12.0Hz, 2H), 7.90(s, 2H), 7.63(s, 2H), 7.24(d, J=9.0Hz, 4H), 6.89(d, J=9.0Hz, 4H), 5.75(s, 2H), 3.79(s, 6H), 3.77-3.70(m, 2H), 2.15(s, 6H), 1.57-1.54(m , 12H).HRMS[ESI]calcd for C 44 h 45 N 2 o8 (M+H) + , 729.3170; found, 729.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com