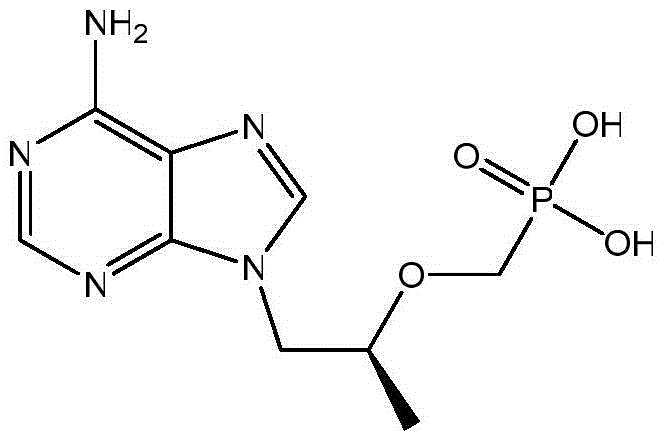
Tenofovir preparation method
An alkyl carbonyl compound technology, applied in the field of preparation of tenofovir, can solve the problems of environmental protection defects of production cost, harsh dissociation conditions, and high operational requirements, and achieve the effects of improving product purity, mild reaction conditions, and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
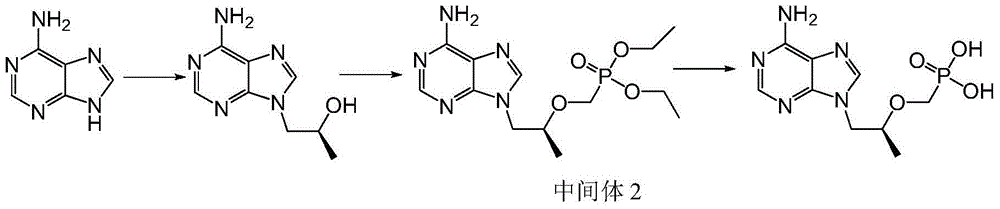
[0040] As shown in Scheme 1, compound III (17.4g, 0.13mol), potassium carbonate (21.5g, 0.15mol), R-propylene carbonate (15.8g, 0.15mol) were dissolved in 50mL N,N-dimethylformaldehyde In amide, heat to 90-125°C and keep it warm for 3-6 hours; then cool down to room temperature, add magnesium isopropoxide (15.7g, 0.14mol) under the protection of argon, heat up to 30-45°C and keep it warm for 0.5-1 Hours; then add diethyl p-toluenesulfonyloxymethylphosphonate (45.9g, 0.15mol) dropwise, and continue the heat preservation reaction at 30-45°C for 4-6 hours; finally, the system is lowered to room temperature, and the reaction system is concentrated under reduced pressure , to intermediate II (as viscous oil).
[0041] Add 124g of concentrated hydrochloric acid to intermediate II, raise the temperature to 90-110°C and keep it warm for 3-6 hours; then cool down to room temperature, continue the reaction at room temperature for 1-2 hours, filter with suction, and filter th...
Embodiment 2
[0043]
[0044] As shown in Scheme 2, compound III (17.4g, 0.13mol), potassium carbonate (21.5g, 0.15mol), R-propylene carbonate (15.8g, 0.15mol) were dissolved in 50mL N,N-dimethylform In amide, heat to 90-125°C and keep it warm for 3-6 hours; then cool down to room temperature, add magnesium isopropoxide (15.7g, 0.14mol) under the protection of argon, heat up to 30-45°C and keep it warm for 0.5-1 Hours; then add dimethyl p-toluenesulfonyloxymethylphosphonate (44.1g, 0.15mol) dropwise, and continue the heat preservation reaction at 30-45°C for 4-6 hours; finally, the system is lowered to room temperature, and the reaction system is concentrated under reduced pressure , to intermediate II (as viscous oil).
[0045]Add 96g of concentrated hydrochloric acid to intermediate II, raise the temperature to 90-110°C, and keep it warm for 3-6 hours; then lower it to room temperature, continue the reaction at room temperature for 1-2 hours, filter with suction, and filter the cake wi...
Embodiment 3
[0047]
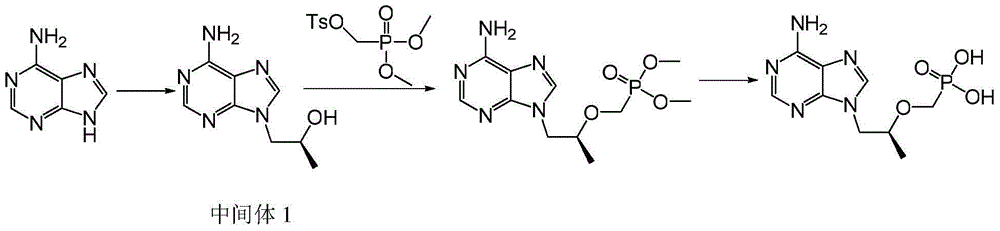
[0048] As shown in Scheme 3, compound III (17.4g, 0.13mol), potassium carbonate (21.5g, 0.15mol), R-propylene carbonate (15.8g, 0.15mol) were dissolved in 50mL N,N-dimethylform In amide, heat to 90-125°C and keep it warm for 3-6 hours; then cool down to room temperature, add magnesium isopropoxide (15.7g, 0.14mol) under the protection of argon, heat up to 30-45°C and keep it warm for 0.5-1 hour; then add diethyl p-toluenesulfonyloxymethylphosphonate (45.9g, 0.15mol) dropwise, and continue the heat preservation reaction at 30-45°C for 4-6 hours; finally lower the system to room temperature, add 50mL of dilute hydrochloric acid and stir After 3 hours, the reaction system was concentrated under reduced pressure to obtain Intermediate II (as a viscous oil).
[0049] Add 96g of concentrated hydrochloric acid to intermediate II, raise the temperature to 90-110°C and keep it warm for 3-6 hours; then cool down to room temperature, continue the reaction at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com