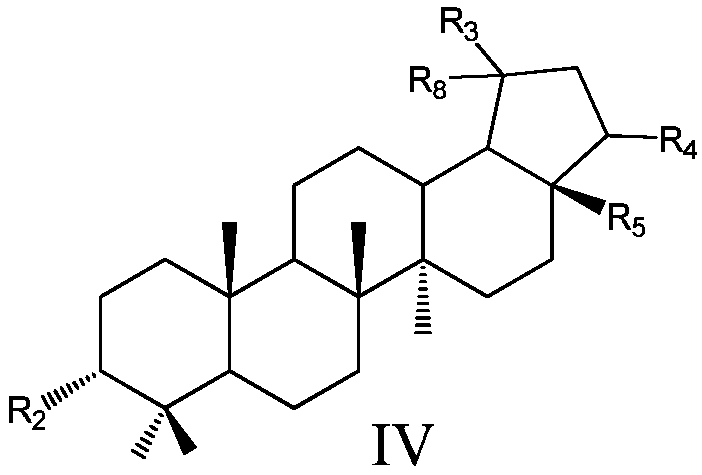
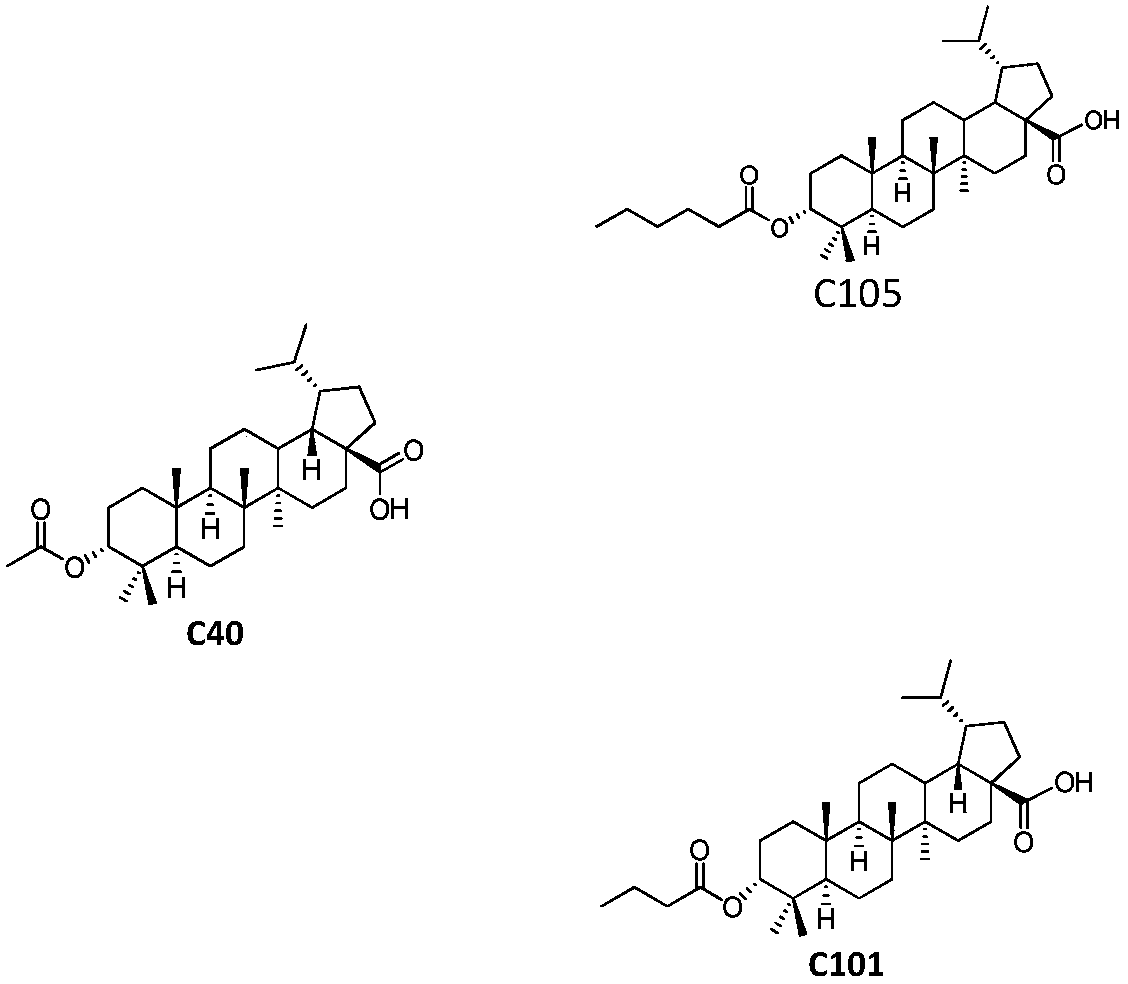
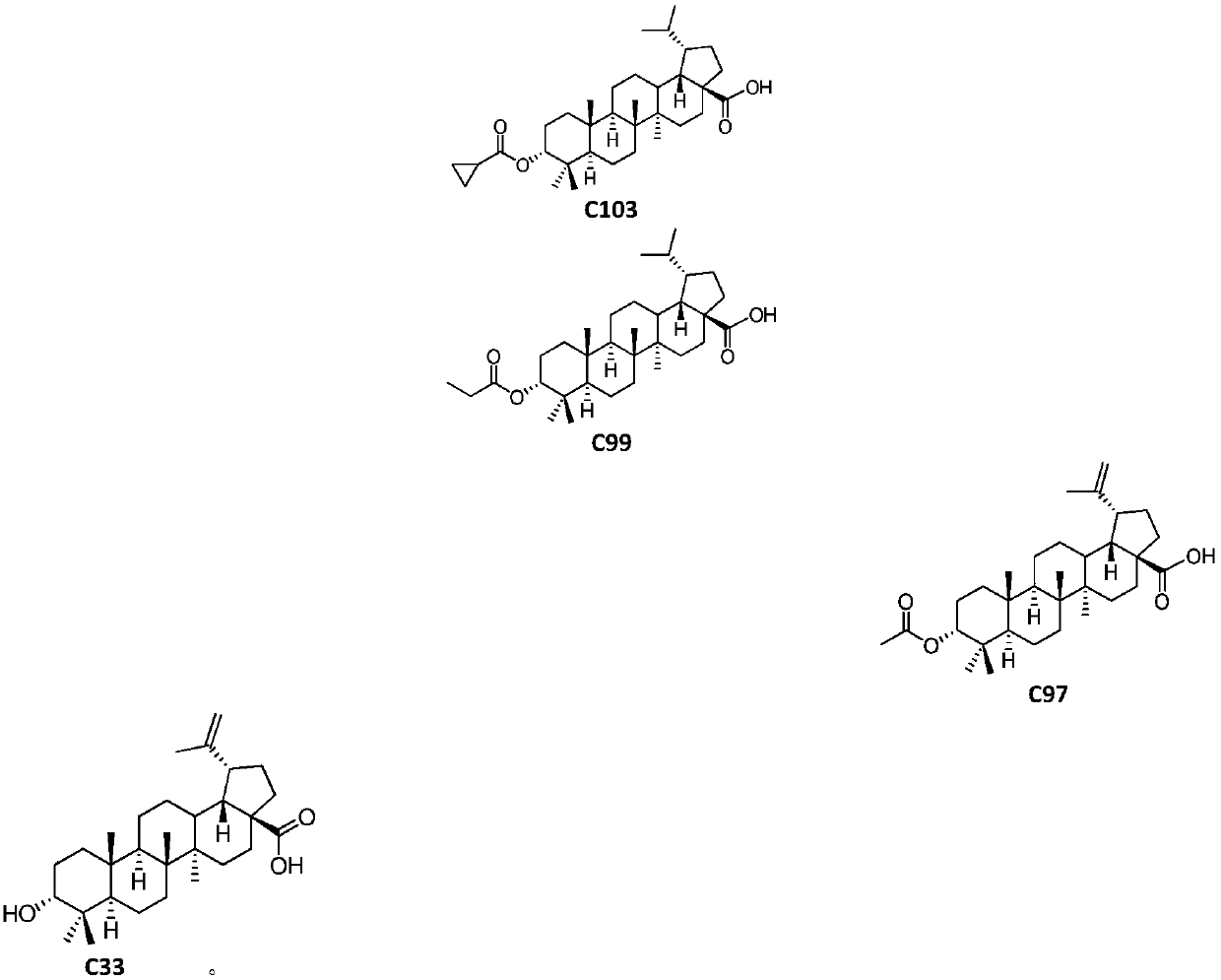
Pentacyclic triterpenoids, preparation method, pharmaceutical composition and use thereof
A compound and drug technology, applied in the field of TGR5 agonists, can solve problems such as preparation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0069] Preparation Example 1 (Compound No.: C33)
[0070]
[0071] (1) Benzyl betulinate
[0072] Dissolve the raw material betulinic acid (4g, 8.76mmol) (purchased from Xi'an Haoxuan Biotechnology Co., Ltd.) in DMF (50mL) at room temperature, add anhydrous potassium carbonate (2.4g, 17.37mmol), and slowly drop it under stirring After adding benzyl chloride (1.2 mL, 10.52 mmol) dropwise, the reaction solution was moved to 50° C. and stirred overnight. The next day, the mixture was cooled to room temperature, diluted with 100 mL of deionized water, extracted with ethyl acetate (2×100 mL), and the combined organic layers were washed with deionized water and saturated brine, dried over sodium sulfate and reduced Pressure distillation gave the desired white solid compound benzyl betulinate (4.62 g), molar yield: 97%. 1 H NMR (300MHz, CDCl 3 )δ7.34(m,5H),5.09(d,1H,J=11.7Hz),5.17(d,1H,J=11.7Hz),4.75(s,1H),4.62(s,1H),3.21- 3.15(m,1H),2.92-2.80(m,1H),2.10-1.90(m,2H),1.87-1.69(m...
preparation Embodiment 2
[0079] Preparation example two (compound number: C34)
[0080]
[0081] Betulinic acid (1.2g, 2.63mmol) was dissolved in methanol / ethyl acetate (40mL / 10mL). After changing the nitrogen, add a catalytic amount of Pd / C, and then change the nitrogen and hydrogen. Stir at room temperature for 2 days, and detect the reaction by TLC completely. After changing the nitrogen gas, the reaction solution was filtered, spin-dried, and separated by column chromatography with petroleum ether:ethyl acetate at a polarity of 10:1. The product C34 was obtained as a white solid (1.04 g, 2.27 mmol) with a molar yield of 86%. 1 H NMR (300MHz, CDCl 3 )δ3.13(t, 1H, J=9.0, 6.9Hz), 2.28-2.16(m, 2H), 1.98-1.78(m, 4H), 1.64-0.96(m, other alicyclic protons), 0.96(s ,3H),0.93(s,3H),0.92(s,3H),0.90(s,3H),0.89(s,3H),0.87(s,3H),0.78(s,3H); ESI-MS( m / z):481.3(M+Na) + (C 30 h 50 o 3 Theoretical value: 458.38).
[0082] Using the same method, using different natural products or compounds as raw mater...
preparation Embodiment 3
[0085] Preparation Example 3 3-α-Acetoxy Betulinic Acid (Compound No.: C97)
[0086]
[0087] (1) Benzyl 3-α-acetoxybetulinate
[0088] 3-α-Hydroxybetulinate benzyl ester (107mg, 0.20mmol) and a catalytic amount of DMAP (10mg, 0.08mmol) were dissolved in dichloromethane (10mL), triethylamine (82mL, 0.60mmol) was added, and ice-water bath Acetic anhydride (42 mL, 0.60 mmol) was added dropwise and reacted at room temperature for 12 hours. TLC showed that the reaction was complete. After concentrating to remove the solvent, diluting with ethyl acetate, washing with water and saturated aqueous sodium chloride solution respectively, drying and concentrating the organic phase, and purifying the residue by column chromatography with petroleum ether / ethyl acetate as the eluent of 20:1 The compound 3-α-acetoxy betulinate benzyl ester was obtained as white solid (77 mg, 0.13 mmol), molar yield: 65%. 1 H NMR (300MHz, CDCl 3 )δ7.34(m,5H),5.09(d,1H,J=11.7Hz),5.17(d,1H,J=11.7Hz),4.62(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com