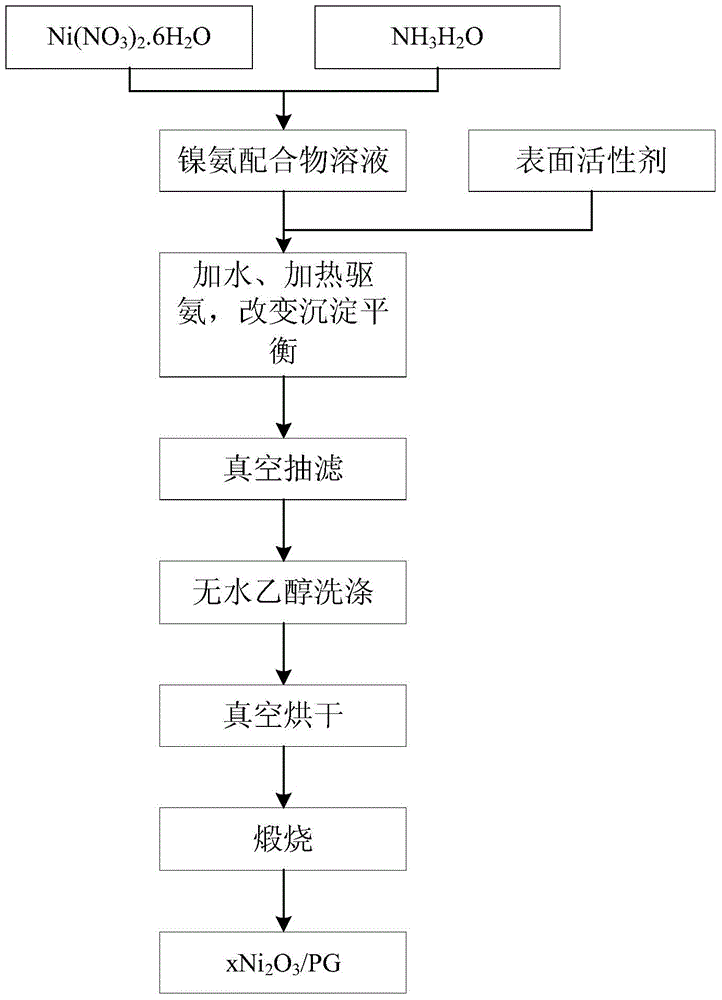
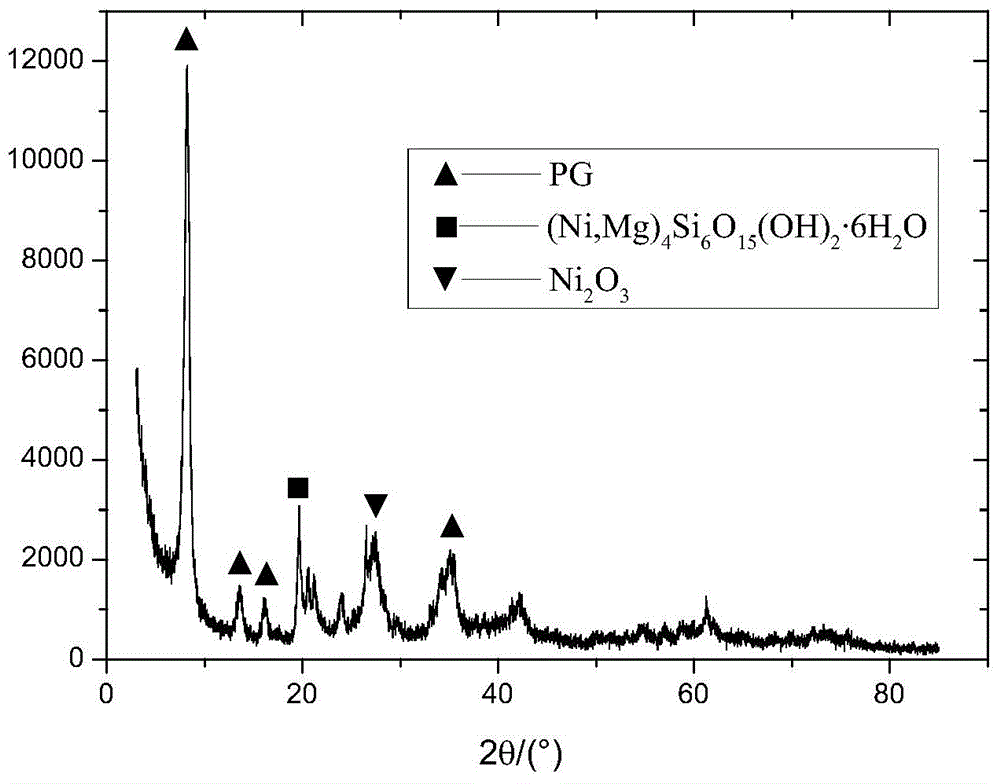
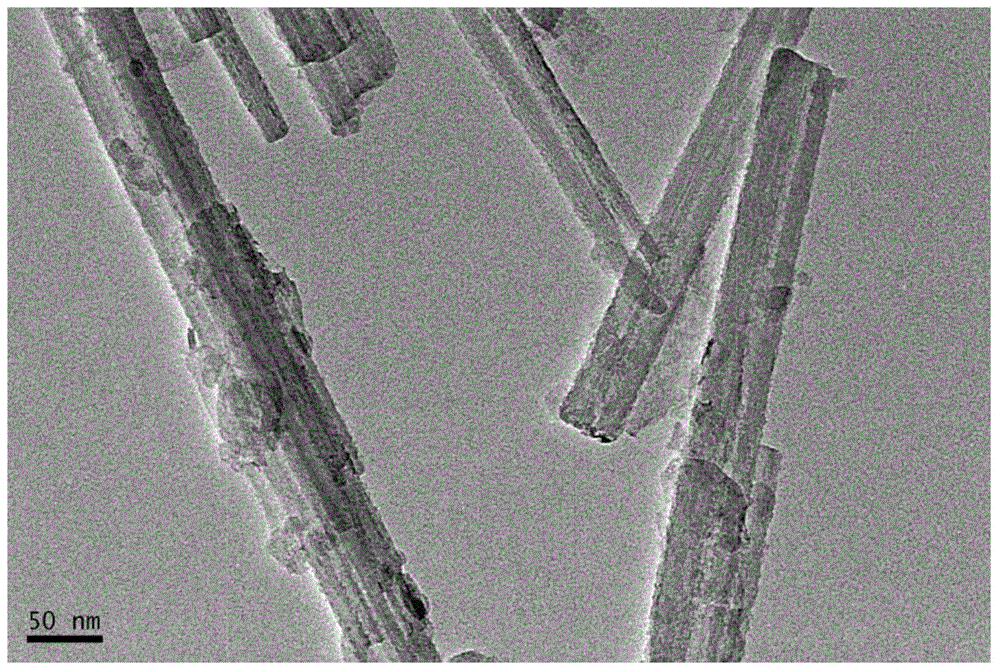
Method for preparing nanometer Ni2O3/PG catalyst with coordination homogenizing precipitation method
A technology of coordinated uniform precipitation and catalyst, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, catalyst carrier, etc., can solve the problem of active component particle agglomeration load, high use cost, regeneration Difficulties and other problems, to achieve the effect of low toxicity, low price, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] [Ni(NH 3 ) 6 ] 2+ The instability constant for is:
[0031] Ni(NO 3 ) 2 .6H 2 O is dissolved in water to obtain a solution. Adding a certain volume of ammonia water with a mass fraction of 28% and a density of 0.87g / ml obtained the Ni 2+ , NH 3 , [Ni(NH3 ) 6 ] 2+ mixed solution. Assuming that after mixing [NH 3 ] is ymol / L, Ni 2+ The concentration is wmol / L, [Ni(NH 3 ) 6 ] 2+ The concentration is xmol / L.
[0032] For Ni(NO 3 ) 2 .6H 2 The reaction of O and ammonia water to form nickel ammonia complex:
[0033]
[0034] The configured volume is V 配 The nickel-ammonia complex solution, assuming the addition of ag Ni(NO 3 ) 2 .6H 2 Ni in solution after O 2+ with [Ni(NH 3 ) 6 ] 2+ The total concentration of is:
[0035]
[0036] Therefore, in solution, Ni 2+ The concentration w of is:
[0037] because
[0038] so:
[0039]
[0040] The relationship between coordination equilibrium and precipitation-dissolution equilibrium can b...
Embodiment 2
[0078] Configure 10Ni 2 O 3 / PG, based on the mass of the carrier attapulgite clay, set to weigh 10g of attapulgite clay as the carrier, so configure 10Ni 2 O 3 / PG requires Ni(NO 3 ) 2 .6H 2 O total 5.5g. Assuming that 100ml of nickel ammonia complex solution is prepared so that Ni 2+ All converted to the complex [Ni(NH 3 ) 6 ] 2+ , then the solution [Ni(NH 3 ) 6 ] 2+ The concentration x is 0.1893mol / L. NH is calculated by 3 The concentration y is 1.2610mol / L. Since the formation of nickel ammonia complexes requires the consumption of 1.1358mol / L of NH 3 , so the required ammonia should contain at least 0.2397mol of NH 3 , that is, at least 16.7ml of ammonia water with a mass fraction of 28% and a relative density of 0.87g / ml is required.
[0079] Since high nickel oxide is a non-stoichiometric compound, the loading is calculated as nickel. Weigh 5.5g of Ni(NO 3 ) 2 .6H 2 O is placed in a 100ml beaker, poured into ultrapure water to prepare a 30ml solutio...
Embodiment 3
[0082] Configure 14Ni 2 O 3 / PG, based on the mass of the carrier attapulgite clay, set to weigh 10g of attapulgite clay as the carrier, so configure 14Ni 2 O 3 / PG requires Ni(NO 3 ) 2 .6H 2 O total 8.1g. Assuming that 100ml of nickel ammonia complex solution is prepared so that Ni 2+ All converted to the complex [Ni(NH 3 ) 6 ] 2+ , then the solution [Ni(NH 3 ) 6 ] 2+ The concentration x is 0.2774mol / L, then the NH 3 The concentration y is 1.3611mol / L. Since the formation of nickel-ammonia complexes requires the consumption of 1.6644mol / L of NH 3 , so the required ammonia should contain at least 0.3026mol of NH 3 , that is, at least 21.1 ml of ammonia water with a mass fraction of 28% and a relative density of 0.87 g / ml is required.
[0083] Since high nickel oxide is a non-stoichiometric compound, the loading is calculated as nickel. Weigh 8.1g of Ni(NO 3 ) 2 .6H 2 O is placed in a 100ml beaker, poured into ultrapure water to prepare a 25ml solution. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com