Fluorescence probe for detecting beta-galactosidase as well as preparation method and application of fluorescence probe
A technology of galactosidase and fluorescent probe, which is applied in the field of analysis and detection, can solve problems such as difficulty in ensuring detection accuracy, and achieve the effects of eliminating background interference, good detection effect, and good detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of probe compound NG-GAL
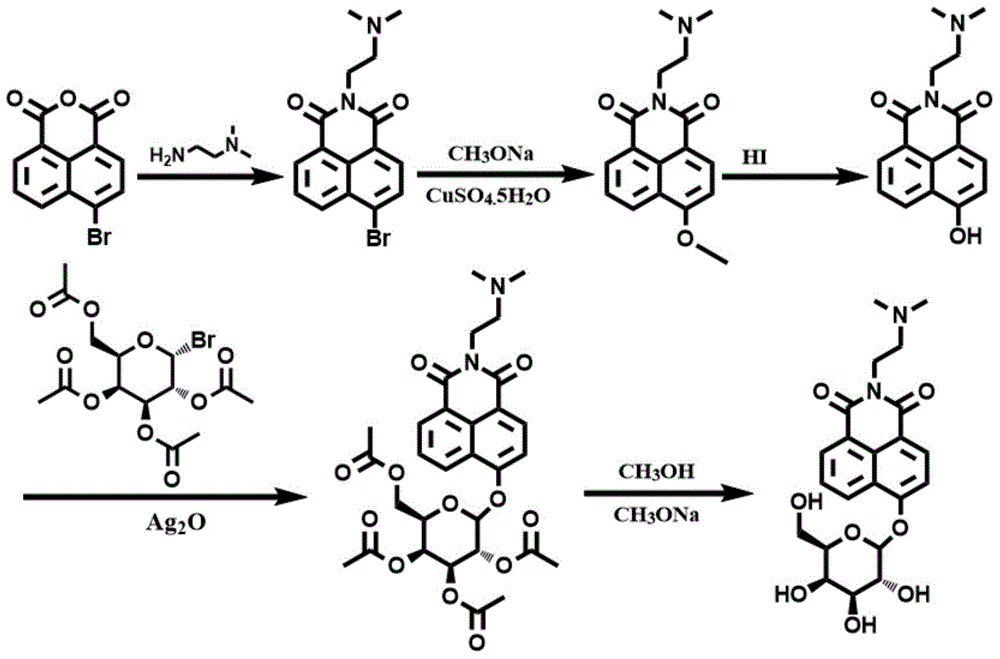
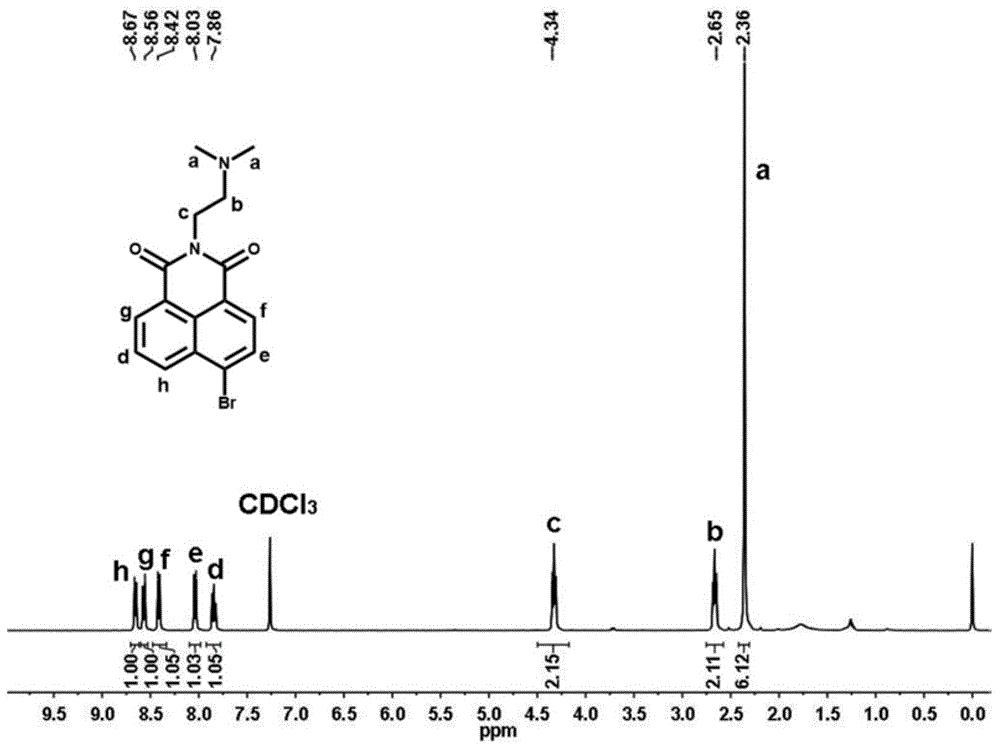
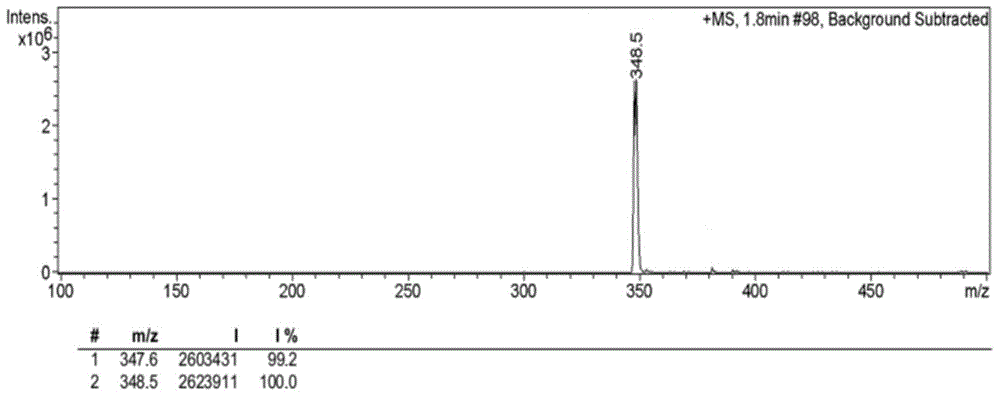
[0064] Synthetic route such as figure 1 As shown, 1035 mg of 4-bromo-1,8-naphthalene dicarboxylic anhydride (3.75 mmol) was dissolved in 56 mL of ethanol, and under stirring conditions, 396 mg of N,N-dimethylethylenediamine was added dropwise under nitrogen protection (4.50mmol), the mixed solution was heated to reflux at 80°C, and after cooling to room temperature, the precipitate was filtered and collected, and the precipitate was recrystallized with ethanol to obtain solid 6-bromo-2-(2-(dimethylamino)ethyl Base)-benzisoquinoline-diketone 1066mg (yield rate is 82.2%); By proton nuclear magnetic resonance spectrum ( figure 2 ) to characterize the product, 1 H NMR (CDCl 3 ,400MHz,ppm):2.36(s,6H),2.65(t,J=6.4Hz,2H),4.34(t,J=6.8Hz,2H),7.86(t,J=6.8Hz,1H),8.03 (d,1H),8.42(d,1H),8.56(d,1H),8.67(d,1H). Among them, 8.67ppm, 8.56ppm, 8.42ppm, 8.03ppm and 7.86ppm correspond to the protons on the naphthalene ring 4.34...
Embodiment 2
[0069] Embodiment 2: the preparation of probe compound NG-GAL
[0070] Dissolve 2070mg of 4-bromo-1,8-naphthalene dicarboxylic anhydride (7.50mmol) in 90mL of ethanol, and add 760mg of N,N-dimethylethylenediamine (8.62mmol ), the mixed solution was heated to reflux at 82°C, and after cooling to room temperature, the precipitate was filtered and collected, and the precipitate was recrystallized with ethanol to obtain solid 6-bromo-2-(2-(dimethylamino)ethyl)- Benzisoquinoline-dione 1988 mg (76.6% yield).
[0071] Dissolve 1038mg of the above white solid (3.0mmol) and 1620mg of sodium methoxide (30mmol) in 21mL of methanol, add 102mg of CuSO 4 ·5H 2 O, under stirring conditions, the solution was heated to reflux at 72°C and maintained for 11 hours; cooled to room temperature, added deionized water, and then extracted with ethyl acetate; the organic phase was collected, and the organic phase was dried with 1800 mg of anhydrous magnesium sulfate, Filtration, rotary evaporation t...
Embodiment 3
[0076] Embodiment 3: the preparation of probe compound NG-GAL
[0077] Dissolve 552mg of 4-bromo-1,8-naphthalene dicarboxylic anhydride (2.0mmol) in 30mL of ethanol, and add 194mg of N,N-dimethylethylenediamine (2.2mmol ), the mixed solution was heated to reflux at 85°C, and after cooling to room temperature, the precipitate was filtered and collected, and the precipitate was recrystallized with ethanol to obtain solid 6-bromo-2-(2-(dimethylamino)ethyl)- Benzisoquinoline-dione 535 mg (76.7% yield).
[0078] Dissolve 346mg of the above white solid (1.0mmol) and 486mg of sodium methoxide (9mmol) in 9mL of methanol, add 34mg of CuSO 4 ·5H 2 O, under stirring conditions, the solution was heated to reflux at 68°C and kept for 11 hours; cooled to room temperature, added deionized water, and then extracted with ethyl acetate; collected the organic phase, and dried the organic phase with 1000 mg of anhydrous magnesium sulfate, Filtration, rotary evaporation to remove the organic so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com