Riemerella anatipestifer OmpA/MotB truncated recombinant protein, antibody and preparation method and application thereof
A technology of Riemeria anatipestifer and recombinant protein is applied in the field of genetic engineering to achieve standardization and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
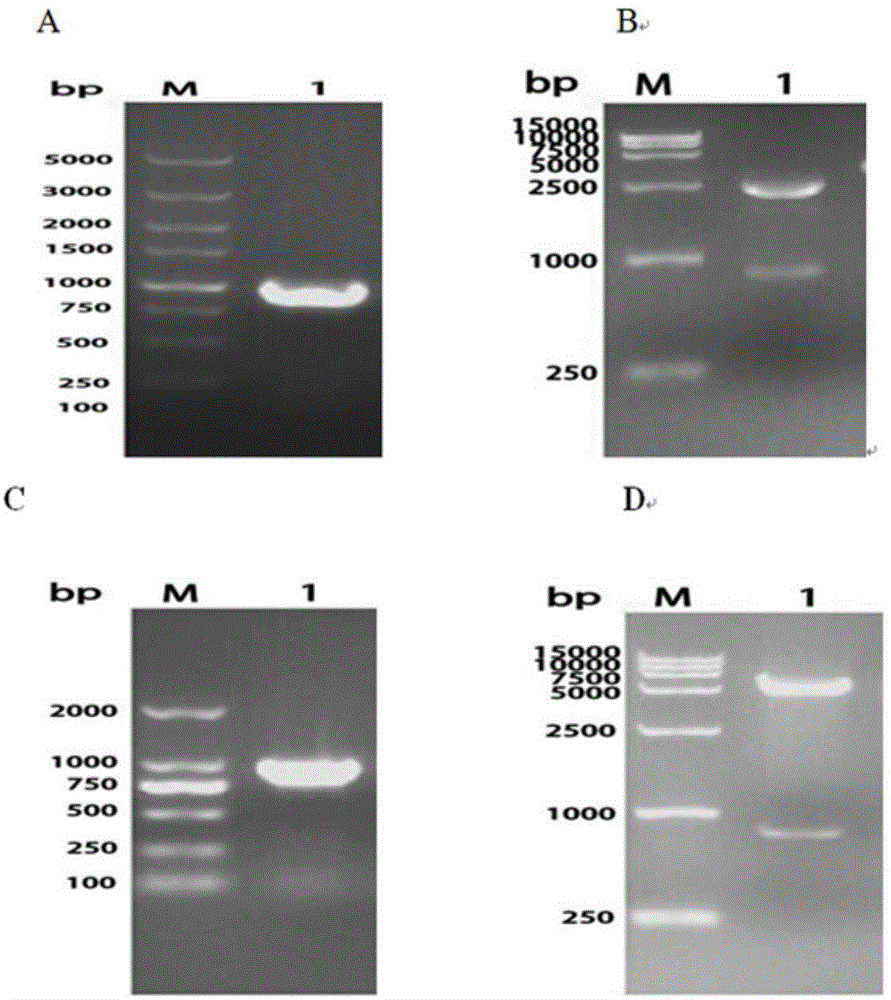
[0031] Example 1 Preparation of truncated OmpA / MotB recombinant protein
[0032] 1. Primer design of OmpA / MotB gene (main epitope region)
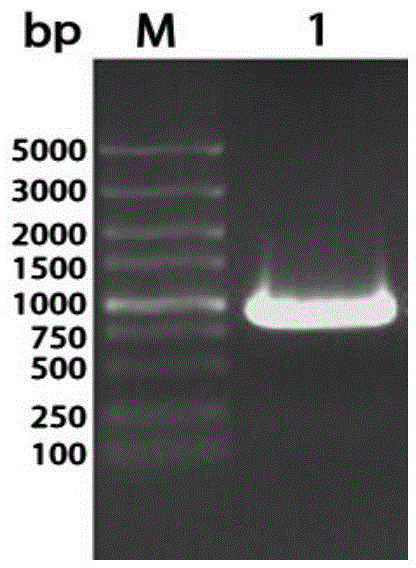
[0033] According to the bioinformatics analysis of RA-CH-1 published by Gene Bank and the predicted OmpA / MotB gene sequence (Genbank ID number: CP003787.1), the OmpA / MotB gene fragment is 1467bp long. Bit analysis, further use the Protein in DNAstar7.0 to carry out antigen analysis on the sequence, and select the main epitope segment of RA-CH-1 OmpA / MotB: 409bp-1389bp segment (969bp) for truncated expression. Using the extracted RA-CH-1 genomic DNA as a template, amplified by PCR, the DNA fragment of the main antigenic epitope region of the OmpA / MotB gene was obtained, the expected length (primer region plus restriction site) 981bp, the main antigen of OmpA / MotB The nucleotide sequence of the epitope region is shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID NO.2.
[0034] PCR cloning primers:
[0035] Upstream prime...
Embodiment 2
[0059] Example 2 Establishment of an indirect ELISA method based on OmpA / MotB truncated protein
[0060] 1. Optimization of reaction conditions based on OmpA / MotB truncated protein ELISA method
[0061] The optimized conditions are shown in Table 1.
[0062] Table 1 Optimization of indirect ELISA conditions
[0063]
[0064]
[0065] Follow the steps below to optimize each reaction condition in Table 1:
[0066] (1) Dilute the protein with the coating solution, add 100 μL to each well of the microplate, overnight at 4°C;
[0067] (2) Discard the liquid the next day, add 200 μL of PBST containing 1% BSA to each well, and block at 37°C for 1 h;
[0068] (3) Wash 4 times with PBST, 5 min each time, pat dry, add 100 μL of serum to be tested, and incubate at 37°C for 1 h;
[0069] (4) Wash 4 times with PBST, pat dry, add 100 μL of HRP-labeled goat anti-duck IgG at working concentration, and incubate at 37°C for 1 h;
[0070] (5) Wash the plate as above, pat dry, add 100 ...
Embodiment 3
[0117] Example 3 Preparation of rabbit anti-OmpA / MotB truncated recombinant protein hyperimmune serum
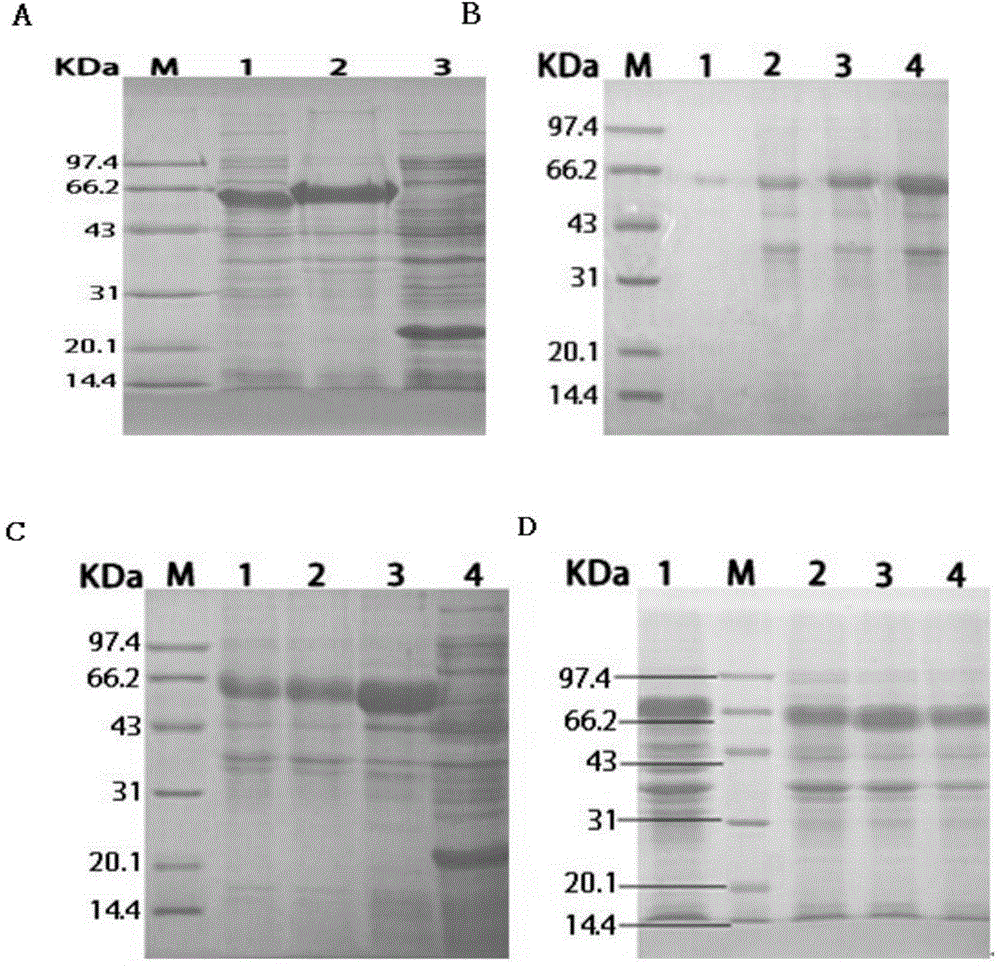
[0118] 1. Preparation of immunogen
[0119] The OmpA / MotB truncated recombinant protein prepared in Example 1 was mixed with Freund's complete adjuvant (FCA) or Freund's incomplete adjuvant (FICA) in equal volumes, placed on an ice bath, and ultrasonically emulsified to form a water-in-oil agent until. Check the emulsification effect standard, that is, drop the emulsified antigen in ice water to form a complete and continuous non-diffusion round oil droplet and keep it for 1 minute.
[0120] 2. Animal immunity
[0121] Rabbits were immunized according to the immunization procedure in Table 16, blood was drawn from the rabbits one day before the first immunization, and serum was separated as negative serum control.
[0122] Table 16 Preparation of hyperimmune serum for rabbit anti-OmpA / MotB truncated recombinant protein immunization program
[0123]
[0124] 3. Prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com