Method for separating and determining rivaroxaban and impurities thereof, and application thereof
A technology for rivaroxaban and impurities, which is applied in the field of analytical chemistry, can solve the problem of not being able to separate and detect rivaroxaban and related impurities at the same time, and achieves a simple and feasible method, strong specificity, and ensured quality controllable. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 The method for separation and determination of rivaroxaban and its impurities
[0050] (1) Take an appropriate amount of rivaroxaban and an impurity reference substance, and dissolve the sample with 30% acetonitrile aqueous solution to obtain a sample solution;
[0051](2) Preparation of mobile phase A: Weigh 2.0 g of sodium pentane sulfonate, pipette 2.0 ml of phosphoric acid in a 1000 ml volumetric flask, add water to dissolve and dilute to the mark, and adjust the pH value to 4.0±0.1; mobile phase B is acetonitrile;
[0052] (3) Take 10 μl of the sample solution of step (1) and inject it into a chromatograph whose model is Shimadzu LC-20AT. The chromatographic column model is Purospher Star RP-18. The temperature of the column oven is 30°C;
[0053] Table 1 The volume of mobile phase A and mobile phase B for linear gradient elution
[0054] time (min)
[0055] Linear gradient elution was performed according to the data shown in Table 1, and ...
Embodiment 2
[0056] Embodiment 2 Chromatographic detection of rivaroxaban and its impurities
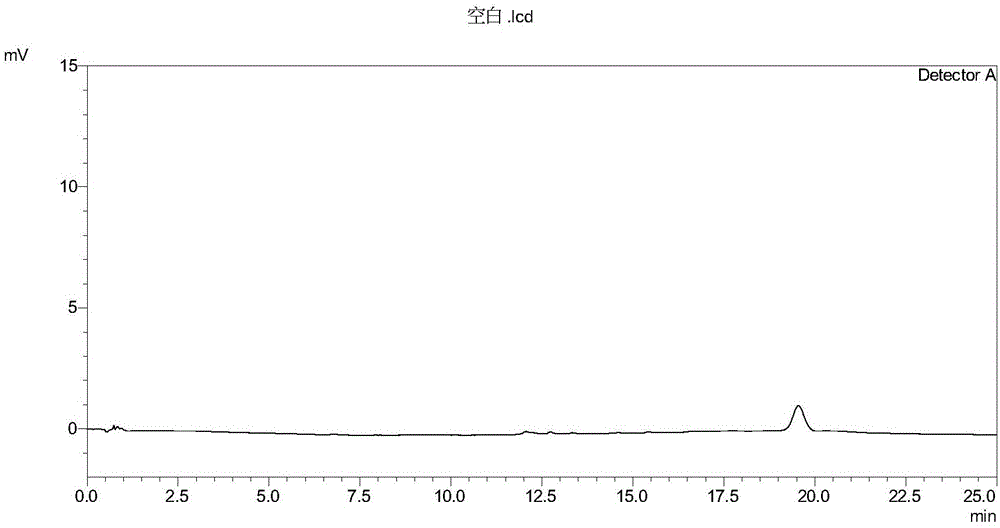
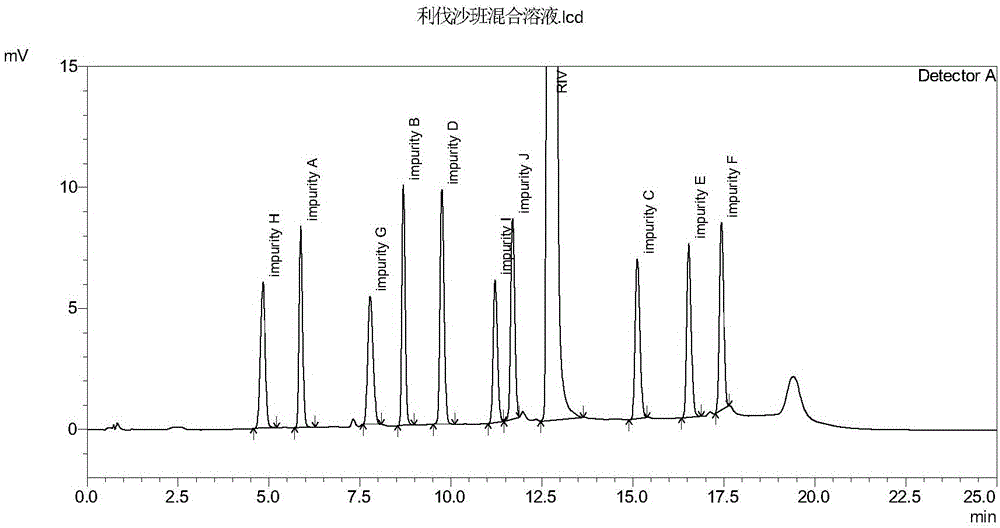
[0057] The diluent was used as a blank control sample, and the sample solution described in step 1) in Example 1 was used as a test sample. Take the diluent and the sample solution respectively and inject samples according to the chromatographic conditions of the method in Example 1, record the chromatogram, and the measurement results are shown in Table 2. High performance liquid chromatography see figure 1 , figure 2 .
[0058] Table 2 test results
[0059]
[0060] The test results show that the diluent does not interfere with the detection of the test product; the number of theoretical plates of each main impurity peak is greater than 10,000; the degree of separation between each chromatographic peak is greater than 1.5; it can be seen that the method has high column efficiency and good separation effect. Strong specificity.
Embodiment 3
[0061] Embodiment 3 does not add acidic ion pair reagent comparative example
[0062] A phosphate buffer solution without acidic ion-pairing reagent was used as the mobile phase A, acetonitrile was used as the mobile phase B, the detection wavelength was 250nm, and the flow rate was 1.0ml / min, and the gradient program in the following table was used for elution:
[0063] time (min)
0
13.0
13.1
18.0
Mobile phase A(%)
92
49
92
92
Mobile phase B(%)
8
51
8
8
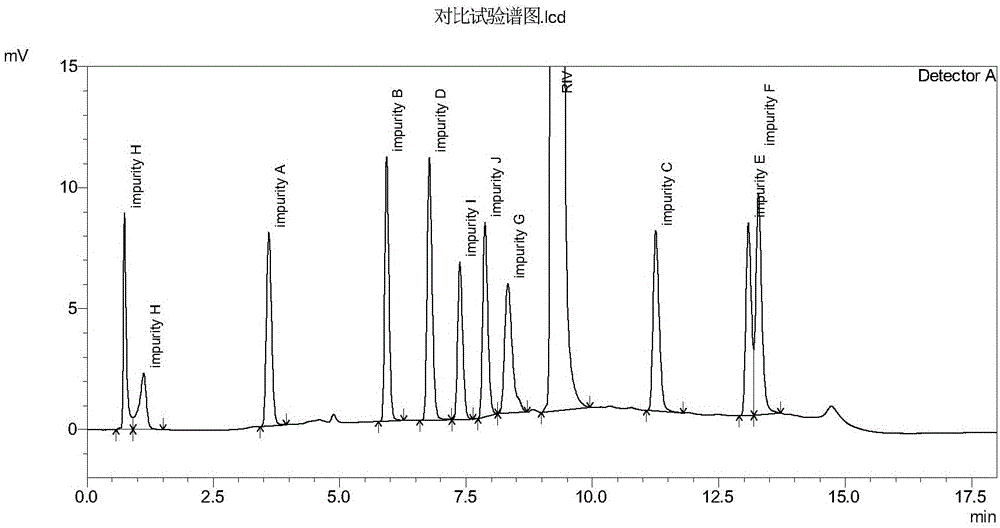
[0064] Get the sample solution described in step 1) in embodiment 1, record the chromatogram, the results are shown in image 3 .
[0065] The test results show that under the chromatographic conditions in the comparative example, impurity H is a split peak, and impurity E and impurity F cannot be effectively separated, which affects the accurate quantitative detection of impurities. The results of comparative examples show that the present inven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com