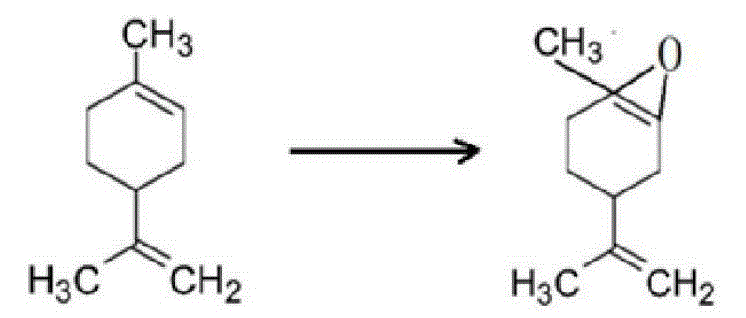
Catalytic synthesis method for limonene-1,2-epoxide
A technology of epoxide and limonene, applied in the direction of organic chemistry, etc., can solve the problems of long cycle, difficult epoxidation of olefins, low synthesis yield of limonene-1,2-epoxide, etc., achieving high efficiency and reducing Environmental pollution, the effect of fast catalytic reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Preparation of borotungstic heteropolyacid catalyst
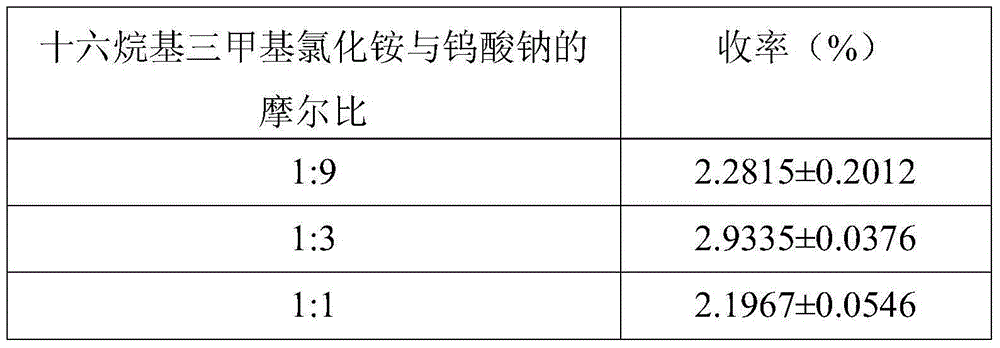
[0024] Weigh 16.491g of sodium tungstate dihydrate, dissolve it in 100ml of distilled water, and stir evenly to form a 0.5mol / L sodium tungstate solution. Add dilute sulfuric acid to the sodium tungstate solution, so that the molar ratio of sulfuric acid to sodium tungstate is 1.5:1. After the reaction, a pale yellow tungstic acid precipitate is obtained. Slowly add 30% H in the light yellow turbid solution 2 o 2 Solution until the precipitate just disappears, and a clear and transparent light yellow peroxytungstic acid solution is obtained. Weigh an appropriate amount of boric acid solid (the molar amount of boric acid is 1 / 3 of that of sodium tungstate), transfer it and peroxytungstic acid solution into a three-necked flask, and react at 60°C for 0.5h to obtain borotungstic heteropoly acid solution. Add hexadecyltrimethylammonium chloride (1 / 3 of the molar weight of sodium tungstate) and react at 80°C for 1 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com