Kit for detecting respiratory virus and its application
A kit and respiratory technology, applied in the field of kits for the detection of respiratory viruses, can solve the problems of time-consuming virus, complex and expensive electron microscopy detection methods, false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, preparation and use thereof for detecting the kit of respiratory tract virus
[0038] 1. Preparation of kits for detecting respiratory viruses
[0039] The kit for detecting respiratory virus provided by the present invention consists of the following:
[0040] 1. Constant temperature amplification buffer
[0041] The solvent of constant temperature amplification buffer is water, solute and concentration are as follows: 200mM Tris-HCL (pH 8.0), 50mM DTT, 10mM dNTP, 10mM rNTP, 80mM MgCl 2 , 450mM KCl, 15% by volume DMSO, 1M sorbitol, 20mM tetramethylammonium chloride.
[0042] 2. Constant temperature amplification enzyme solution
[0043] The solvent of the constant temperature amplification enzyme solution is water, and the solute and concentration are as follows: AMV reverse transcriptase 1U / μl, T7 RNA polymerase 5U / μl, ribonuclease H 0.5U / μl, pyrophosphatase 0.5U / μl, RNase inhibitor Agent 5U / μl, BSA0.5μg / μl.
[0044] 3. A 24-chamber disc chip loaded...
Embodiment 2
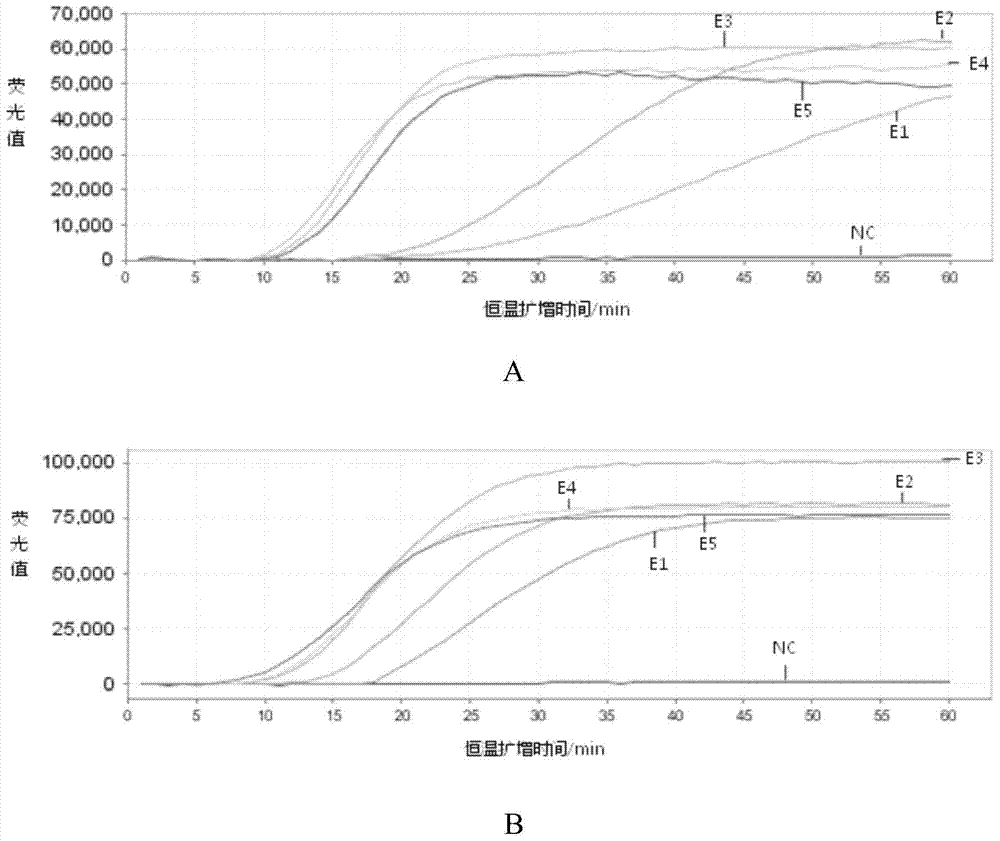
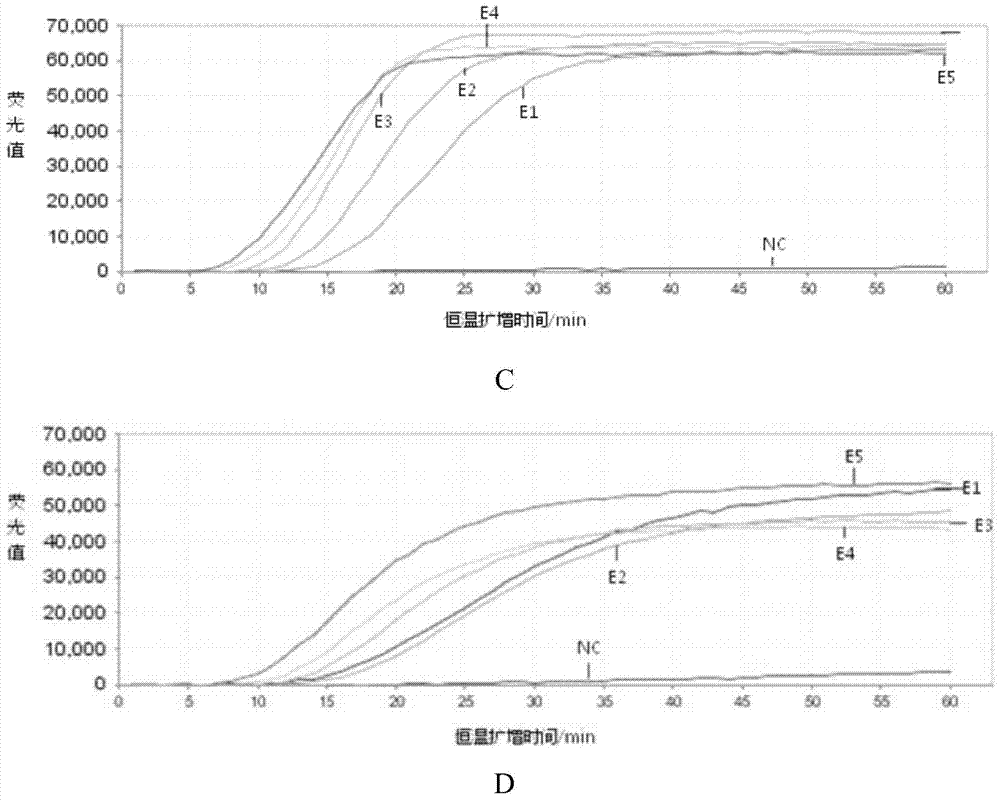
[0058] Example 2. Sensitivity and specificity analysis of the kit for detecting respiratory viruses
[0059] 1. Preparation of reference RNA nucleic acid
[0060] 1. Construction of plasmids containing respiratory virus target genes
[0061] (1) Plasmids containing respiratory syncytial virus target genes
[0062] Insert the segment 5680-7390 of the RSV target gene sequence (Genbank number Sequence ID of the RSV target gene sequence: gb|KJ627648.1|, Update Date: 2014-4-16) into the pUC19 vector (day With the multiple cloning site EcoRⅤ of the biochemical company product), the recombinant plasmid pUC19-RSV was obtained.
[0063] (2) Plasmids containing rhinovirus target genes
[0064] Insert the segment 1-1175 of the rhinovirus target gene sequence (Genbank number Sequence ID: gb|M16248.1|, Update Date: 2003-2-7) of the rhinovirus target gene sequence into the pUC19 vector (product of Tiange Biochemical Company) ) between the multiple cloning sites EcoRⅤ to obtain the recom...
Embodiment 3
[0090] Embodiment 3, the detection of actual clinical sample
[0091] 1. Types of clinical samples
[0092] The clinical samples used in this example came from the nasopharyngeal swab samples collected by Shenzhen No. 3 Hospital (based on the principle of voluntariness of the collectors), and the collected swabs were stored in 3 mL of normal saline, a total of 560 cases.
[0093] 2. Extraction of viral nucleic acid in clinical samples
[0094] The kit used for the extraction of viral nucleic acid from clinical samples is QIAamp Viral RNA Mini Kit (Qiagen), and the extraction is performed as follows:
[0095] (1) Take 140 μl of physiological saline for storing clinical sample swabs in step 1 into a 1.5ml centrifuge tube;
[0096] (2) Add 560 μl Buffer AVL containing Carrier RNA mixture (i.e. 5.6 μl Carrier RNA mixture + 560 μl Buffer AVL to the centrifuge tube, vortex slightly for 15 seconds;
[0097] (3) After the transient centrifugation is completed, place it at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com