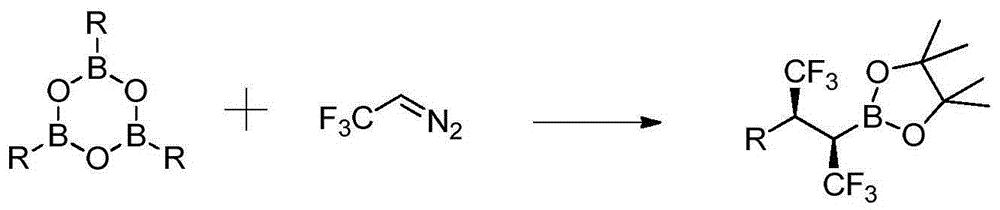
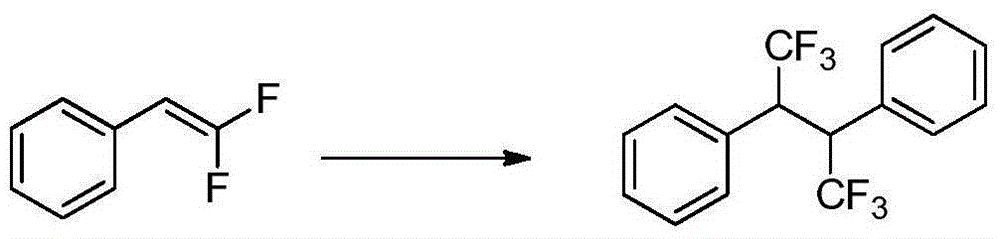
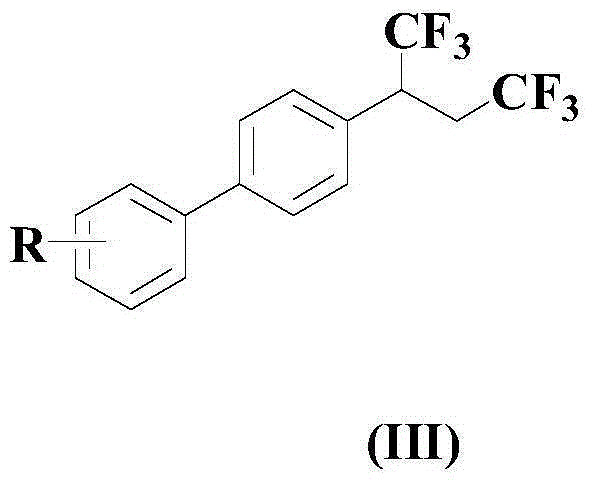
Trifluoromethylation method for biphenylyl olefin compound
A technology of compounds and synthetic methods, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation, etc., can solve the problems of low material conversion rate and long reaction time, and achieve good application prospects and industrial production potential. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Under the protection of nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of polyethylene glycol and 15-crown-5 with a volume ratio of 1:0.2) at room temperature, 100mmol of the compound of the above formula (I) and 250mmol of the above formula (II) ) Compound, 5mmol catalyst (1.25mmolPd(dba) 2 And 3.75mmol ytterbium trifluoromethanesulfonate), 10mmol oxidant silver acetate and 20mmol N-n-butyl-N-methylpiperidine bis(trifluoromethanesulfonyl)imide salt, and then heated to 50℃ , And the reaction was stirred at this temperature for 10 hours.
[0044] After the reaction is complete, filter, adjust the pH of the filtrate to neutral, then wash with saturated sodium bicarbonate aqueous solution, add dichloromethane for extraction, separate the organic phase, dry with anhydrous sodium sulfate, concentrate under reduced pressure, and leave the residue After 200-300 mesh silica gel column chromatography, using a mixture of petroleum ether and acetone in a ...
Embodiment 2
[0046] Under the protection of a nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of polyethylene glycol and 15-crown-5 with a volume ratio of 1:0.2) at room temperature, 100mmol of the compound of the above formula (I) and 200mmol of the above formula (II) ) Compound, 7mmol catalyst (2mmolPd(dba) 2 And 5mmol ytterbium trifluoromethanesulfonate), 15mmol oxidizer silver acetate and 25mmol accelerator N-n-butyl-N-methylpiperidine bis(trifluoromethanesulfonyl)imide salt, and then heated to 60°C, The reaction was stirred at this temperature for 8 hours.
[0047] After the reaction is complete, filter, adjust the pH of the filtrate to neutral, then wash with saturated sodium bicarbonate aqueous solution, add dichloromethane for extraction, separate the organic phase, dry with anhydrous sodium sulfate, concentrate under reduced pressure, and leave the residue After 200-300 mesh silica gel column chromatography, using a mixture of petroleum ether and acetone w...
Embodiment 3
[0049] Under the protection of nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of polyethylene glycol and 15-crown-5 with a volume ratio of 1:0.2) at room temperature, 100mmol of the compound of the above formula (I) and 250mmol of the above formula (II) ) Compound, 9mmol catalyst (being 3mmolPd(dba) 2 And 6mmol ytterbium trifluoromethanesulfonate), 12mmol oxidizer silver acetate and 30mmol accelerator N-n-butyl-N-methylpiperidine bis(trifluoromethanesulfonyl)imide salt, and then heated to 70°C, The reaction was stirred at this temperature for 6 hours.
[0050] After the reaction is complete, filter, adjust the pH of the filtrate to neutral, then wash with saturated sodium bicarbonate aqueous solution, add dichloromethane for extraction, separate the organic phase, dry with anhydrous sodium sulfate, concentrate under reduced pressure, and leave the residue After 200-300 mesh silica gel column chromatography, using a mixture of petroleum ether and aceto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com