Double targeting liposome with 4-amino-benzene-alpha-D-mannopyranoside (MAN) and wheat germ agglutinin (WGA) modifiers and preparation method thereof and application
A technology targeting liposomes and liposomes, applied in liposome delivery, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of undetected epirubicin, epirubicin, Doxorubicin can not reach the therapeutic effect of glioma and other problems, so as to improve the effect of chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the preparation of liposome
[0059] 1. Preparation of blank liposomes
[0060] 1. Preparation of blank liposomes
[0061] Egg yolk lecithin, cholesterol, DSPE-PEG 2000 、DSPE-PEG 2000 -COOH and DSPE-PEG 2000 -NH 2 Dissolve in methanol at a molar ratio of 52:43:4:0.5:0.5, remove methanol by rotary evaporation, and form a film in an eggplant-shaped flask by rotary evaporation at 35°C and 40rpm, then add 250mM ammonium sulfate aqueous solution, and then perform ultrasonic treatment (ultrasonic parameters : Every 10s of work, stop for 30s, the whole time is 10min, and the protection temperature is 35°C), then use a liposome extruder to pass through the 400nm polycarbonate membrane 3 times and the 200nm polycarbonate membrane 3 times (the sequence is as follows: 400nm polycarbonate membrane Carbon lipid film → 400nm polycarbonate film → 400nm polycarbonate film → 200nm polycarbonate film → 200nm polycarbonate film → 200nm polycarbonate film), then packed i...
Embodiment 2
[0084] Embodiment 2, the performance measurement of liposome
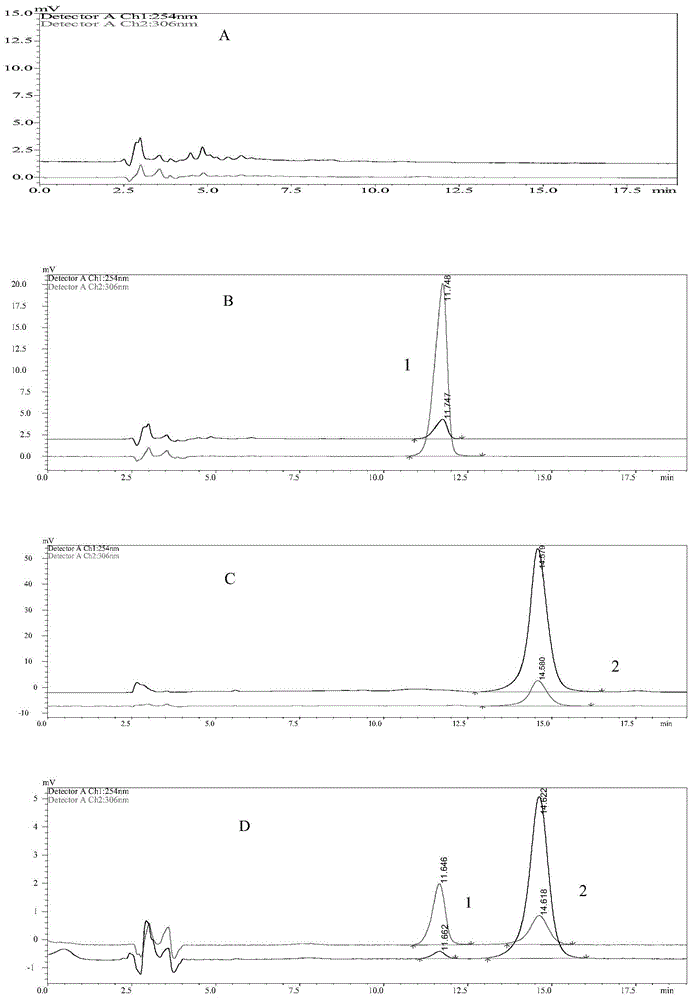
[0085] 1. Determination of Encapsulation Efficiency
[0086] Liposome A1, liposome A2, liposome B1, liposome C1, and liposome D1 obtained in Example 1 were respectively subjected to the following (1) or (2) operations.
[0087] (1) Liposome A1, liposome A2, liposome B1, liposome C1, and liposome D1 were respectively subjected to the following operations: liposomes were placed in a 10mL volumetric flask, and 10 times the volume of methanol was used to Destroy (obtain demulsification solution), detect the concentration of resveratrol and epirubicin.
[0088] (2) Liposome A1, liposome A2, liposome B1, liposome C1, and liposome D1 were respectively subjected to the following operations: the liposomes were passed through a shephedexG-50 column, buffered with PBS of pH 7.4 solution to remove free resveratrol, then packed in a dialysis bag and dialyzed in PBS buffer at pH 7.4 to remove free epirubicin, and then placed i...
Embodiment 3
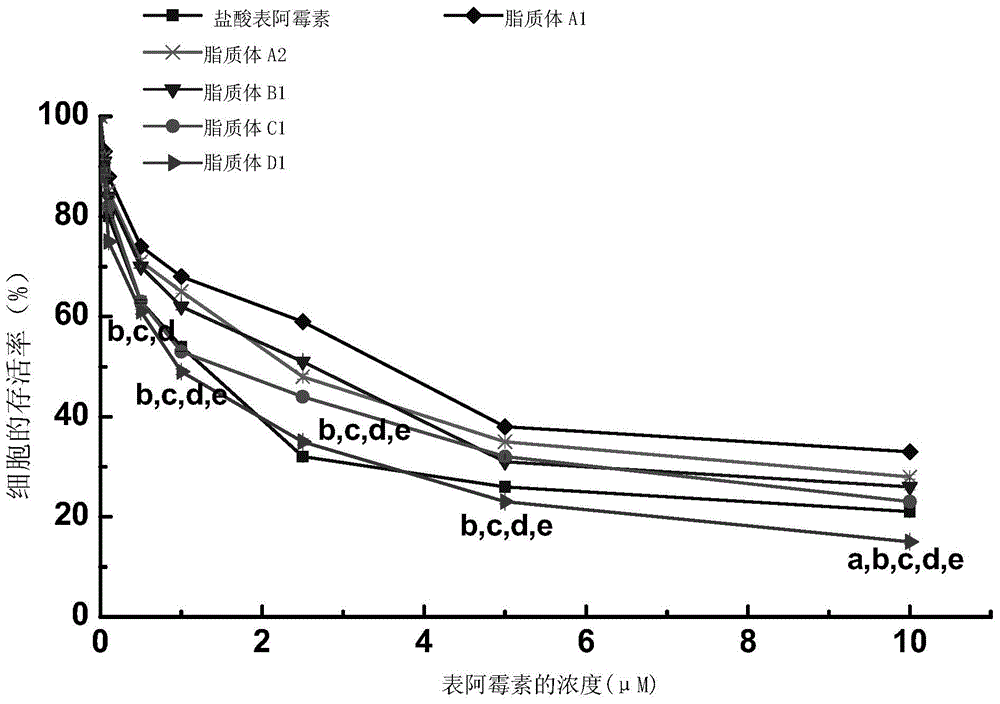
[0098] Example 3. Research on the inhibitory effect of drug-loaded liposomes on C6 glioma cells in vitro
[0099] When the added form of medicine is liposome, the added amount of epirubicin hydrochloride is the added amount of liposome multiplied by the encapsulation efficiency.
[0100] 1. Take C6 rat brain glioma cells in the logarithmic growth phase, inoculate 3000 cells / well (190 μL / well) in a 96-well plate, place at 37°C, 5% CO 2 Conditioned for 24h.
[0101]2. After completing step 1, take the 96-well plate, and add 10 μL of drug solutions with serial concentrations prepared by PBS buffer solution of pH 7.4 to each well (the addition forms of the drugs are respectively: epirubicin hydrochloride, liposome A1, liposome A2, liposome B1, liposome C1 or liposome D1, make the concentration of each test group epirubicin hydrochloride be 0.05, 0.1, 0.5, 1, 2.5, 5 or 10 μ M respectively; Use an equal volume of PBS buffer to replace the blank control of the drug solution) at 37°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com