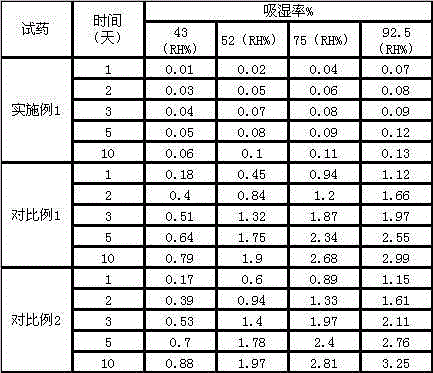
Ranitidine hydrochloride composition freeze-dried powder injection for treating stomach illness
A technology of ranitidine hydrochloride and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of ranitidine hydrochloride is easy to deliquescence, drug effect decline, color darkening, etc., and achieves low content of insoluble particles and good stability , good fluidity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of ranitidine hydrochloride crystals
[0029] (1) Dissolve ranitidine hydrochloride in a mixed solvent of water and dimethyl sulfoxide, the required amount of solvent per gram of ranitidine hydrochloride is 90ml, and the volume ratio of water and dimethyl sulfoxide is 4: 1.5;
[0030] (2) After heating to 30°C to dissolve, add seed crystals after cooling to room temperature;
[0031] (3) Cool to below 0°C, stir and crystallize, the crystallization temperature is -10°C, filter, dry, collect crystals to obtain ranitidine hydrochloride crystals.
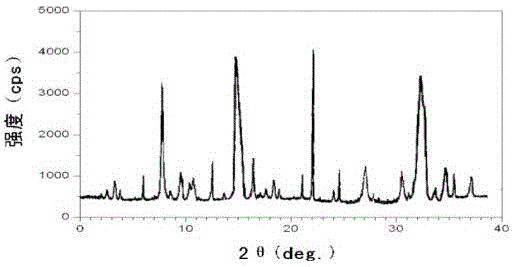
[0032] The X-ray powder diffraction figure that the prepared ranitidine hydrochloride crystal uses Cu-Kα ray measurement to obtain is as follows figure 1 shown.
Embodiment 2
[0033] Example 2: Preparation of ranitidine hydrochloride freeze-dried powder injection
[0034] Prescription: in parts by weight, 1 part of ranitidine hydrochloride crystalline compound prepared in Example 1, and 10 parts of trehalose.
[0035] Preparation method: take the prescribed amount of ranitidine hydrochloride compound, stir and dissolve it with water for injection, add the prescribed amount of excipients, adjust the pH value to 6.0-8.0, then stir until the pH remains constant, then add water for injection to the volume of the solution It is 100 times the weight of ranitidine hydrochloride, then coarsely filtered with activated carbon, sterilized and filtered through 1.0 μm, 0.45 μm, and 0.22 μm microporous membranes in turn, and filtered into a sterile room. Pack it, press half the stopper, put it into a freeze-drying box that has been cooled to -40°C, freeze-dry at low temperature, press the stopper out of the box, and roll the cap.
[0036] Preferably, the excip...
Embodiment 3
[0041] Example 3: Preparation of ranitidine hydrochloride freeze-dried powder injection
[0042] Prescription: in parts by weight, 1 part of ranitidine hydrochloride crystalline compound prepared in Example 1, 11 parts of trehalose.
[0043] Preparation method: take the prescribed amount of ranitidine hydrochloride compound, stir and dissolve it with water for injection, add the prescribed amount of excipients, adjust the pH value to 6.0-8.0, then stir until the pH remains constant, then add water for injection to the volume of the solution It is 100 times the weight of ranitidine hydrochloride, then coarsely filtered with activated carbon, sterilized and filtered through 1.0 μm, 0.45 μm, and 0.22 μm microporous membranes in turn, and filtered into a sterile room. Pack it, press half the stopper, put it into a freeze-drying box that has been cooled to -40°C, freeze-dry at low temperature, press the stopper out of the box, and roll the cap.
[0044] Preferably, the excipient...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com