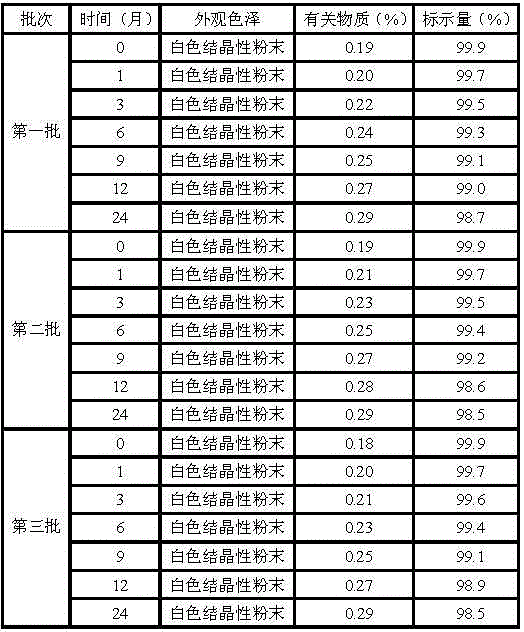
Erdosteine pharmaceutical composition dry suspension for treating diseases of respiratory system
A technology for respiratory diseases and dry suspensions, applied in the field of medicine, can solve the problems of drug absorption speed or degree, low effective bioavailability, slow drug dissolution rate, etc., and achieve high stability, good solubility, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Erdosteine Crystals
[0028] (1) Dissolve erdosteine crystals in a mixed solvent of methanol and isopropyl ether, the required amount of solvent per gram of erdosteine is 110ml, and the volume ratio of methanol to isopropyl ether is 5:3;
[0029] (2) After heating to 50°C to dissolve, add seed crystals after cooling to room temperature;
[0030] (3) Cool to below 0°C, stir and crystallize, the crystallization temperature is -20°C, filter, dry, collect crystals to obtain Erdosteine crystals.
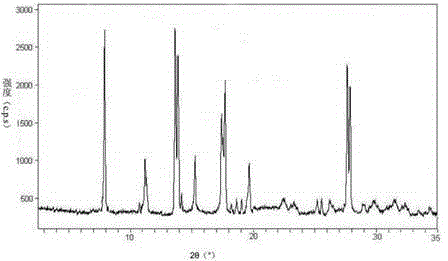
[0031] The X-ray powder diffraction pattern obtained by measuring the obtained erdosteine crystal using Cu-Kα ray is as follows: figure 1 Shown, its purity as determined by high performance liquid chromatography is 99.9%.
Embodiment 2
[0032] Example 2: Preparation of Erdosteine Dry Suspension
[0033] Prescription: in parts by weight, 15 parts of erdosteine prepared in Example 1, 60 parts of sucrose, 19 parts of xylitol, 8 parts of disodium hydrogen phosphate, 4 parts of propylene glycol alginate, 0.5 part of sucralose, 9 parts of purified water.
[0034] Preparation:
[0035] 1) Processing of raw and auxiliary materials: use a pulverizer to pulverize erdosteine, sucrose, xylitol, disodium hydrogen phosphate, and propylene glycol alginate through an 80-mesh sieve;
[0036] 2) Weighing: Weighing according to the process prescription;
[0037] 3) Mixing granulation: Add the prescribed amount of erdosteine, sucrose, xylitol, disodium hydrogen phosphate, propylene glycol alginate, and sucralose into the wet mixing granulator, and turn on the stirring motor to dry mix for 10 Minutes, add the prescribed amount of purified water, wet mix and cut for 100-120 seconds to make soft materials, select 18-mesh s...
Embodiment 3
[0041] Example 3: Preparation of Erdosteine Dry Suspension
[0042] Prescription: in parts by weight, 15 parts of erdosteine prepared in Example 1, 61 parts of sucrose, 20 parts of xylitol, 8.5 parts of disodium hydrogen phosphate, 5 parts of propylene glycol alginate, 1 part of sucralose, 9.2 parts of purified water.
[0043] Preparation:
[0044] 1) Processing of raw and auxiliary materials: use a pulverizer to pulverize erdosteine, sucrose, xylitol, disodium hydrogen phosphate, and propylene glycol alginate through an 80-mesh sieve;
[0045] 2) Weighing: Weighing according to the process prescription;
[0046] 3) Mixing granulation: Add the prescribed amount of erdosteine, sucrose, xylitol, disodium hydrogen phosphate, propylene glycol alginate, and sucralose into the wet mixing granulator, and turn on the stirring motor to dry mix for 10 Minutes, add the prescribed amount of purified water, wet mix and cut for 100-120 seconds to make soft materials, select 18 mesh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com