Preparation method of Olaparib and analogue of Olaparib
The technology of an analog and a substituent is applied in the field of preparation of raw materials, and can solve the problems of expensive palladium catalyst, complicated reaction, unsuitable for industrial production, etc., and achieve the effects of promoting development, easy availability of raw materials, environmental protection and economical process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
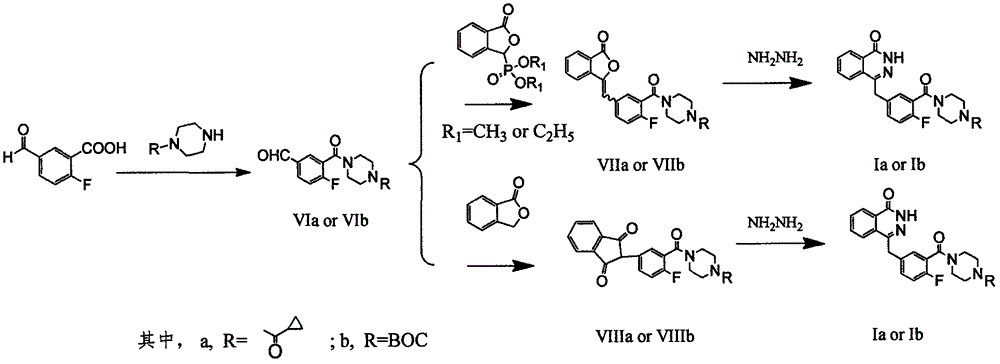
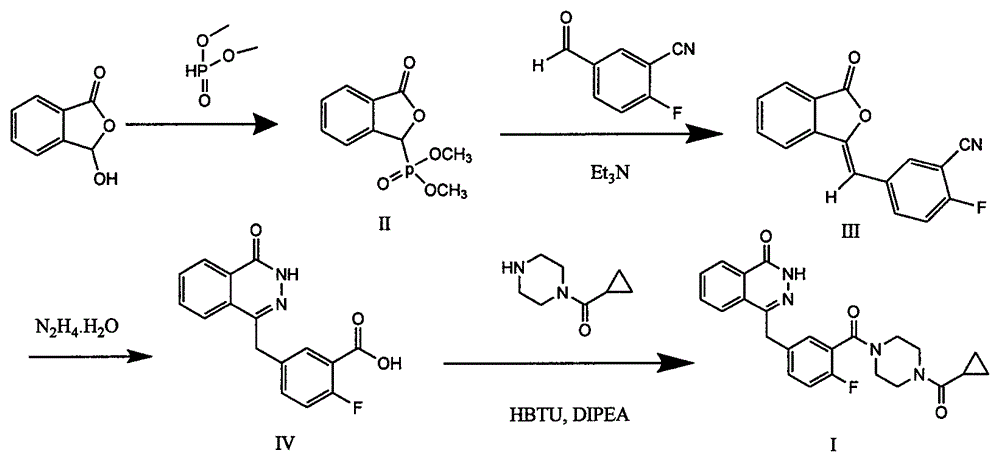
[0043] The preparation method of olaparib comprises the following steps:
[0044] a), add 1.68g of 2-fluoro-5-formylbenzoic acid and 5mL of thionyl chloride into the round bottom flask, add a few drops of N, N-dimethylformamide, and react at room temperature until the raw materials are completely reacted, then reduce The thionyl chloride was removed under pressure to obtain 1.8 g of an oily product, 2-fluoro-5-formylbenzoyl chloride, which was directly used in the next reaction.
[0045] b), 1.65 g of 1-cyclopropanylpiperazine was dissolved in 20 mL of dichloromethane, 1.5 mL of triethylamine was added, and the 2-fluoro-5-formylbenzoyl chloride prepared in the previous step was added dropwise at 0°C in 10 mL The solution of dichloromethane, dropwise, react at room temperature until the raw materials are completely reacted, wash with 1M hydrochloric acid, saturated sodium carbonate, and saturated brine, dry the organic layer, dry over anhydrous sodium sulfate, filter, and conce...
Embodiment 2
[0049] The preparation method of olaparib comprises the following steps:
[0050] a), add 3.4g of 2-fluoro-5-formylbenzoic acid, 30mL of dichloromethane, 3mL of thionyl chloride into the round bottom flask, add dropwise a few drops of N,N-dimethylformamide, and reflux until The reaction of the raw material was complete, and the solvent was removed under reduced pressure to obtain 3.7 g of an oily substance, 2-fluoro-5-formylbenzoyl chloride, which could be directly used in the next reaction.
[0051] Dissolve 3.3 g of 1-cyclopropanoylpiperazine in 20 mL of dioxane, add 4 mL of diisopropylethylamine, and add the 2-fluoro-5-formylbenzoyl chloride prepared in the previous step dropwise at 0°C in 10 mL The solution of dioxane, dropwise, reflux reaction until the reaction of the raw materials is complete, washed with hydrochloric acid, saturated sodium carbonate, and saturated brine to wash the organic layer, dried over anhydrous sodium sulfate, filtered, and concentrated under red...
Embodiment 3
[0055] The preparation method of olaparib comprises the following steps:
[0056] a) Add 1.68g of 2-fluoro-5-formylbenzoic acid, 30mL of dichloromethane and 1g of triphosgene to a round bottom flask, add a few drops of N,N-dimethylformamide, and reflux until the raw materials react Completely, the solvent was removed under reduced pressure to obtain 1.8 g of oily product, 2-fluoro-5-formylbenzoyl chloride, which could be directly used in the next reaction.
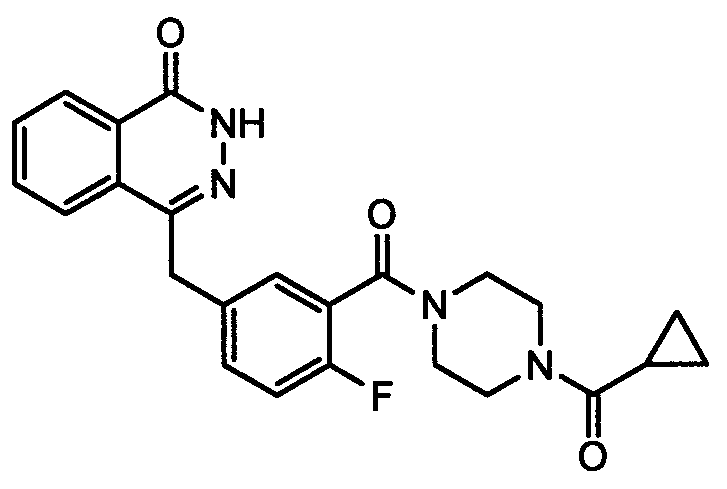
[0057] Dissolve 1.5g of 1-cyclopropanoylpiperazine in 20mL of toluene, add 1.1mL of N-methylmorpholine, and add dropwise at room temperature the solution of 2-fluoro-5-formylbenzoyl chloride dissolved in 10mL of toluene , dropwise, reflux reaction until the raw materials are completely reacted, washed with hydrochloric acid, saturated sodium carbonate, and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain intermediate (VIa): 3-( 4-cyclopropylformyl)piperaziny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com