Nickel-molybdenum carbide composite catalyst for preparing synthesis gas through dry reforming of methane
A methane dry reforming and composite catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, inorganic chemistry, etc., can solve long carbonization time, cumbersome operation, catalyst deactivation, etc. problems, to achieve the effect of increasing the number of catalytic active centers, simplifying the carbonization steps, and reducing sintering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0027] Weigh 25.79 grams of Al(NO 3 ) 3 .9H 2 O and 10.32 g CO(NH 2 ) 2 , add 20 ml of deionized water and mix to form solution #1, and place it in a constant temperature water bath at 80°C under magnetic stirring. Weigh 2.94 g of starch and add 5 mL of deionized water to form solution #2. Weigh 4.25 g (NH 4 ) 6 Mo 7 o 24 .4H 2 O and 1.08 g of citric acid were added to 10 mL of deionized water to form solution #3. Slowly drop #2 into solution #1 to form a homogeneous mixture, then drop into solution #3 and stir for 2.5h to form a gel. Dry at 105°C for 12h, and bake at 550°C for 5h; then in 50ml / min of CH 4 / CO 2 =1 in the mixed gas at 5°C / min to 800°C for carbonization to obtain the CDUT-M5A catalyst, the nitrogen adsorption / desorption test results show that its specific surface area is 32.6m 2 / g. The weight composition of the catalyst is as follows: the content of molybdenum carbide is 12.4%, and the content of aluminum oxide is 87.6%.
[0028] The catalyst wa...
Embodiment 1
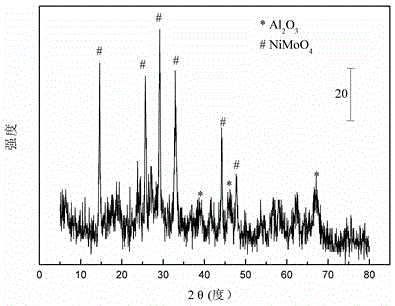
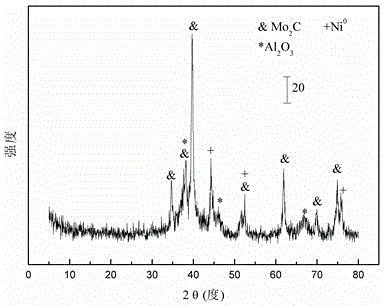
[0030] Weigh 22.56 grams of Al(NO 3 ) 3 .9H 2 O and 9.03 g CO(NH 2 ) 2 , add 20 milliliters of deionized water and mix to form solution #1, place it in a constant temperature water bath at 80°C under magnetic stirring; weigh 2.57 grams of starch, add 5 milliliters of deionized water to form solution #2; weigh 2.81 grams of Ni ( NO 3 ).6H 2 O, 2.23 g (NH 4 ) 6 Mo 7 o 24 .4H 2 0 and 3.41 grams of citric acid, add 10 milliliters of deionized water to form solution #3; #2 is slowly dropped into solution #1 to form a uniform mixture, and solution #3 is added dropwise, and stirred for 2.5h to form a gel; Dry at 105°C for 12h, and bake at 550°C for 5h to obtain the oxide precursor, whose typical nickel-molybdenum oxide structure is as attached figure 1 shown; then, at 50ml / min of CH 4 / CO 2 =1 in the mixed gas at 5°C / min to 800°C for carbonization to obtain CDUT-NM3A catalyst, the typical Ni-Mo 2 C / Al 2 o 3 Composite figure 2 shown. After nitrogen adsorption / desorp...
Embodiment 2
[0033] Weigh 21.63 grams of Al(NO 3 ) 3 .9H 2 O and 8.66 g CO(NH 2 ) 2 , add 20 milliliters of deionized water and mix to form solution #1, place it in a constant temperature water bath at 80°C under magnetic stirring; weigh 2.37 grams of starch, add 5 milliliters of deionized water to form solution #2; weigh 2.52 grams of Ni ( NO 3 ).6H 2 O, 3.56 g (NH 4 ) 6 Mo 7 o 24 .4H 2 0 and 3.63 grams of citric acid, add 10 milliliters of deionized water to form solution #3; #2 is slowly dropped into solution #1 to form a uniform mixture, and solution #3 is added dropwise, and stirred for 2.5h to form a gel; Dry at 105°C for 12h, and bake at 550°C for 5h to obtain the oxide precursor, whose typical nickel-molybdenum oxide structure is as attached figure 1 shown; then, at 50ml / min of CH 4 / CO 2 =1 in the mixed gas at 5°C / min to 800°C for carbonization to obtain CDUT-NM5A catalyst, its typical Ni-Mo 2 C / Al 2 o 3 Composite figure 2 shown. After nitrogen adsorption / desorp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com