Quaternary ammonium salt functionalized porphyrin catalyst and preparation method thereof
A quaternary ammonium salt function and catalyst technology, which is applied in chemical instruments and methods, organic chemistry, cobalt organic compounds, etc., can solve the problems of low molecular weight of copolymers and narrow molecular weight distribution of polymers, shorten the induction period and improve the catalytic effect Good, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
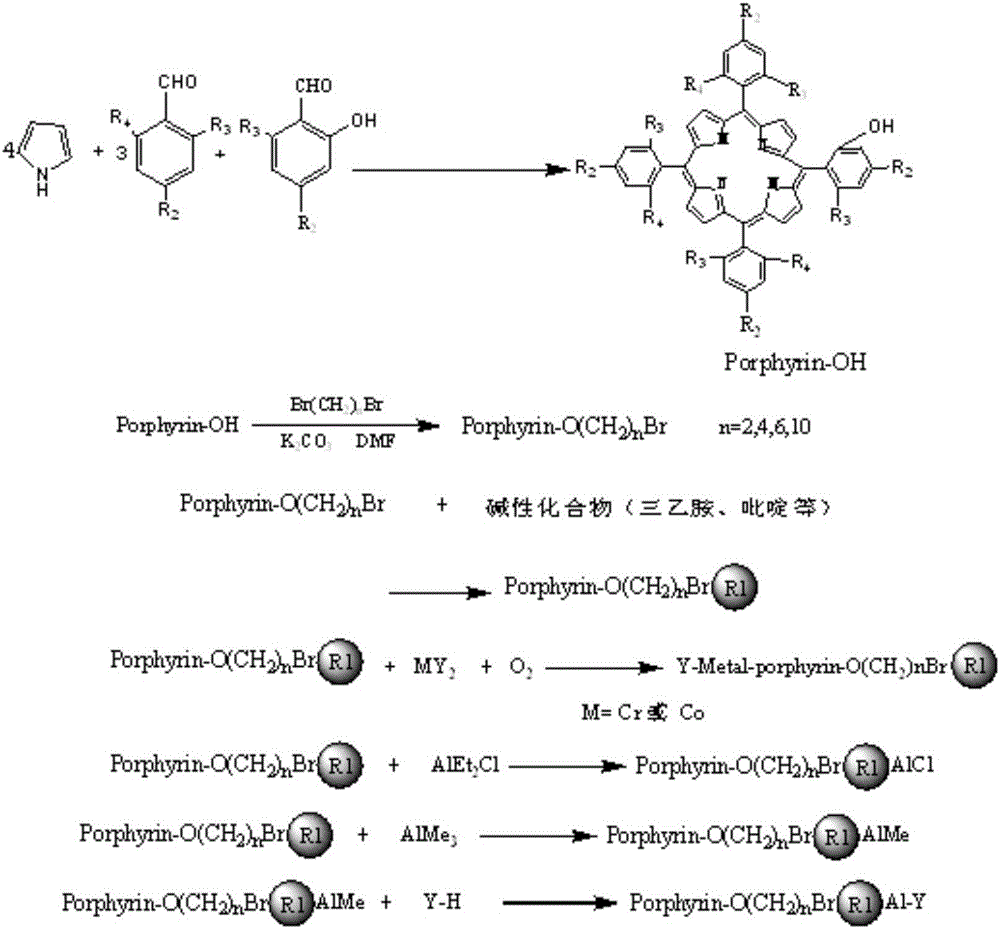
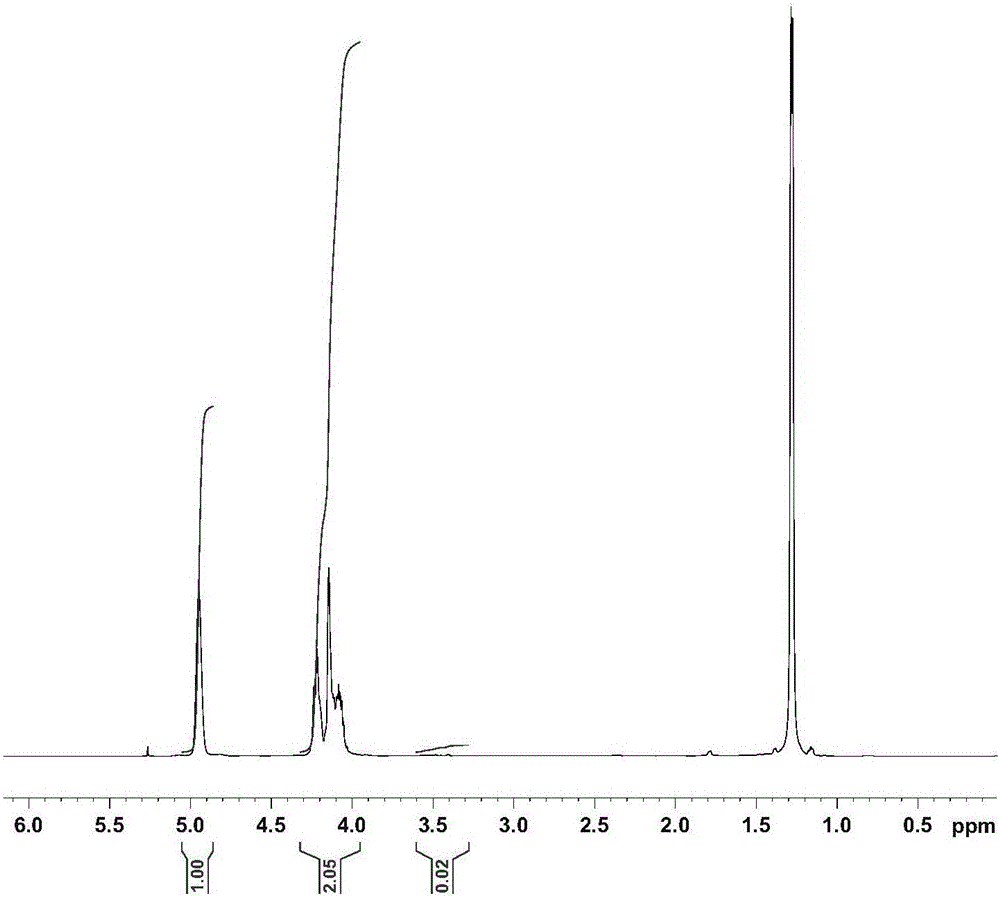
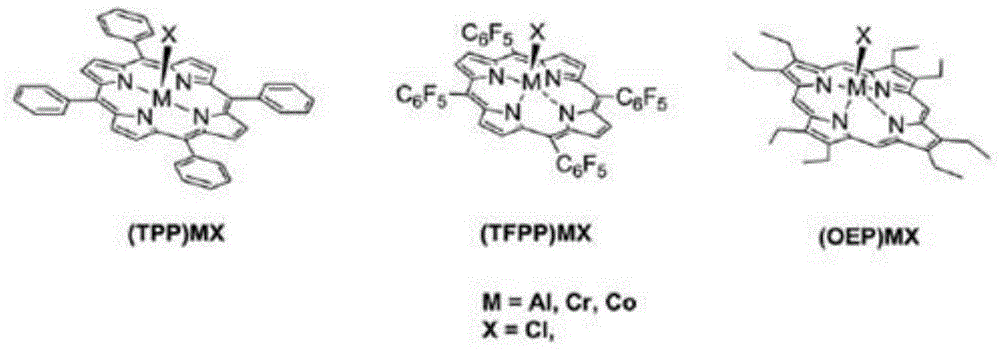
[0030]Catalyst preparation: Take 4.98g (46.9mmol) of benzaldehyde, 3.8g salicylaldehyde (31.3mmol) and 4.2g pyrrole (62.6mmol) (benzaldehyde: salicylaldehyde: pyrrole = 3:2:4, molar ratio) and weigh After measuring, add solvent 150mL propionic acid, reflux reaction at 130-150°C for 4h, concentrate propionic acid to 75mL, add 75mL anhydrous methanol, put in refrigerator at 0°C for 24h, filter, and dry the precipitate in vacuum , after separation by silica gel column chromatography, 5-o-hydroxyphenyl-10,15,20-triphenylporphyrin ((OH)TPPH 2 )), 1.008g (OH)TPPH 2 (1.6mmol), 0.158g1,2-dichloroethane and excess anhydrous potassium carbonate 1.104g ((OH)TPPH 2 : 1,2-dichloroethane:anhydrous potassium carbonate=1:1:5, molar ratio) was added into 100mL N,N-dimethylformamide (DMF), reacted at room temperature for 48h, removed the solvent, added 100mL di Chloromethane is filtered, and the filtrate is extracted with water for 3-5 times, the organic liquid is taken, the solvent is remove...
Embodiment 2
[0035] Prepare catalyst: reactant p-methoxybenzaldehyde 6.38g (46.9mmol), 3.8g salicylaldehyde (31.3mmol) and 4.2g pyrrole (62.6mmol) (benzaldehyde: salicylaldehyde: pyrrole=3:2: 4, (molar ratio)) After weighing, add solvent 150mL propionic acid, reflux at 130-150°C for 6h to obtain crude product, concentrate propionic acid to 75mL, add 75mL anhydrous methanol, place in refrigerator at 0°C Set aside for 24h, filtered, the precipitate was vacuum-dried, and separated by silica gel column chromatography to obtain 5-o-hydroxyphenyl-10,15,20-tri-p-methoxyphenylporphyrin (CH 3 O) 3 (OH)TPPH 2 , 1.15g (CH 3 O) 3 (OH)TPPH 2 (1.6mmol), 0.203g1,4-dichlorobutane and excess anhydrous potassium carbonate 1.1g ((CH 3 O) 3 (OH)TPPH 2 : 1,4-dichlorobutane:potassium carbonate=1:1:5, molar ratio) into 100mL N,N-dimethylformamide (DMF), react at room temperature for 48h, remove solvent, add 100mL dichloromethane and filtered, the filtrate was extracted with water for 3-5 times, the organ...
Embodiment 3
[0040] Catalyst preparation: the same method as in Example 1 was used to prepare 5-o-hydroxyphenyl-10,15,20-phenylporphyrin ((OH)TPPH 2 ), the 1.008g (OH) TPPH 2 (1.6mmol), 0.39g1,6-dibromohexane and excess anhydrous potassium carbonate 1.1g((OH)TPPH 2 : 1,6-dibromohexane:potassium carbonate=1:1:5, molar ratio) into 100mL N,N-dimethylformamide (DMF), react at room temperature for 48h, remove solvent, add 100mL dichloromethane and filtered, the filtrate was extracted with water for 3-5 times, the organic liquid was taken, the solvent was removed, and the bromoporphyrin (O(CH 2 ) 6 Br)TPPH 2 ; Take 1.141g (O(CH 2 ) 6 Br)TPPH 2 (1.44mmol), 0.342g pyridine are dissolved in the mixed solvent 50mL of chloroform and acetonitrile (the volume ratio of chloroform and acetonitrile is 1:1, (O(CH 2 ) 6 Br)TPPH 2 The mol ratio with pyridine is 1:3), reflux reaction 96h, evaporate to dryness and separate through silica gel column chromatography to obtain pyridine loaded porphyrin (O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com