Preparation process of interventional therapy magnetic targeting drug carrier
A technology of interventional therapy and preparation technology, which is applied in the direction of drug combination, non-active ingredient medical preparations, pharmaceutical formulations, etc. It can solve the problem of unsafe reduction of particle agglomeration, negative effects of polymer viscosity, unfavorable drug combination and adhesion, etc. problem, to achieve good stabilization effect, reduce production cost, and achieve the effect of small content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
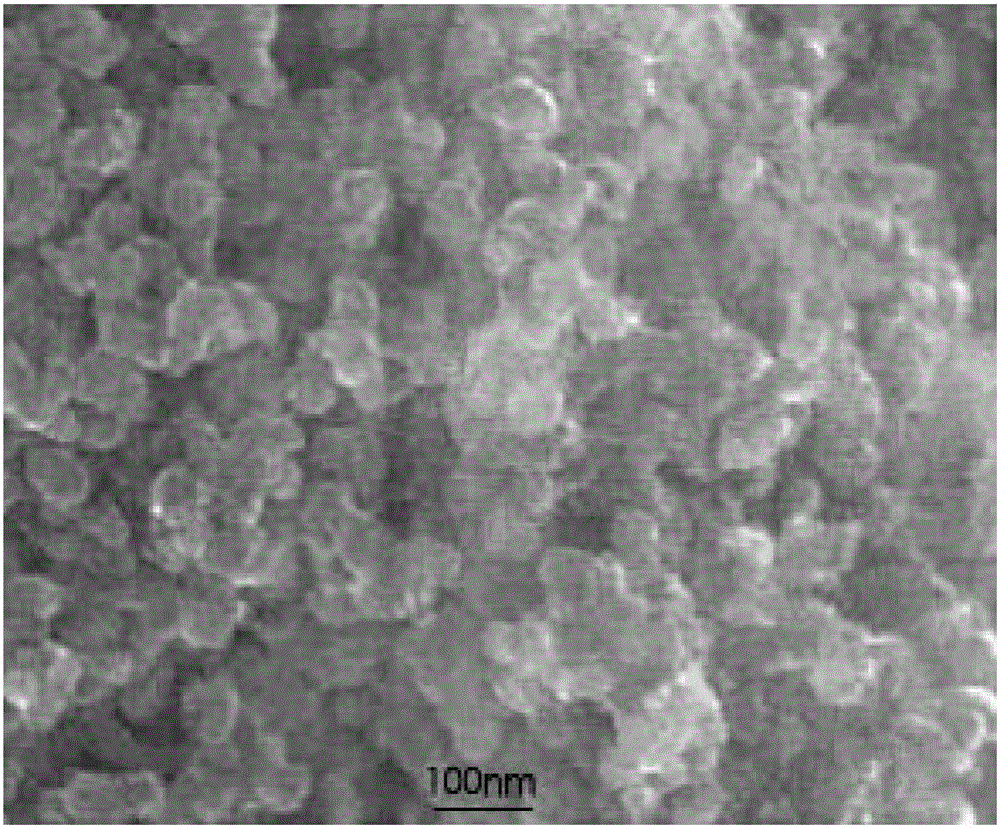
Image
Examples
Embodiment 1
[0023] (1) Weigh 8 moles of NaOH, dissolve in deoxygenated pure water to form a 1.3mol / L solution, add 110g of water-soluble starch, and stir at 25°C until completely dissolved; form a mixed solution; add the mixed solution to the reaction kettle , seal the reactor, use high-purity nitrogen to purge the reactor through the inlet valve, and discharge the internal air; because the soluble starch is added to the hot NaOH solution to undergo gelatinization reaction, the starch molecules in the granules stretch and diffuse in all directions, and the dissolution Outside the granule, the expanded starch molecules will be connected and entangled with each other to form a network of hydrocolloids. When the starch enters the granule disintegration stage of the gelatinization reaction, the viscosity of the solution is the largest, enabling the starch molecules to wrap around NaOH, inhibiting the reaction speed of NaOH with iron ions and ferrous ions, and inhibiting the formation of Fe fro...
Embodiment 2
[0031] (1) Weigh 2 moles of NaOH, dissolve in deoxygenated pure water to form a 1.0mol / L solution, heat to 60°C, add 40g of soluble starch, stir until completely gelatinized, and form a mixed solution; add the mixed solution to Into the reactor, seal the reactor, use high-purity nitrogen to purge the reactor through the inlet valve, and discharge the internal air;
[0032] (2) Weigh 0.25 moles of FeCl 3 ·6H 2 O, 0.25 mol FeCl 2 4H 2 O is dissolved in deoxygenated pure water to form a mixed solution; its concentration is 1.0mol Fe per liter of solution 3+ and 1.0molFe 2+ .
[0033] (3) Add the mixed solution obtained in step (2) dropwise to the sodium hydroxide starch gelatinization solution obtained in step (1) through a feed valve. The proportion of raw materials added is molar ratio OH — : Fe 3+ : Fe 2+ =8.0:1.0:1.0, heat the reactor to 180°C, keep it warm for 10 hours, after cooling down naturally, wash and dry to obtain carbon-coated nano-magnetic Fe 3 o 4 .
...
Embodiment 3
[0036] (1) First weigh 8 moles of NaOH, dissolve it in deoxygenated pure water to form a 1.2mol / L solution, add 80g of pregelatinized starch at 40°C, and stir until completely dissolved; ultrasonically mix at a frequency of 200Hz, and ultrasonically Make the air bubbles in the mixture vibrate under the action of sound waves, and when they reach a certain limit, they will grow and collapse to play a role of cavitation. Using ultrasonic cavitation, NaOH and starch molecules in the solution are evenly mixed and the size of starch particles is controlled. evenly distributed. Add the mixed solution into the reaction kettle, seal the reaction kettle, use high-purity nitrogen to purge the reaction kettle through the inlet valve, and discharge the internal air;
[0037] (2) Weigh 1.5 moles of FeCl 3 ·6H 2 O, 1 mole FeCl 2 4H 2 O is dissolved in deoxygenated pure water to form a mixed solution; its concentration is 1.5mol Fe per liter of solution 3+ and 1.0molFe 2+ .
[0038] (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com