One-pot method for synthesizing N-substituted phthalimide through carbonylation
A phthalimide, carbonylation reaction technology, applied in organic chemistry and other directions, can solve problems such as poor atom economy, high-pressure reaction conditions, etc., and achieve the effects of excellent yield, simple operation, and good adaptability of functional groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
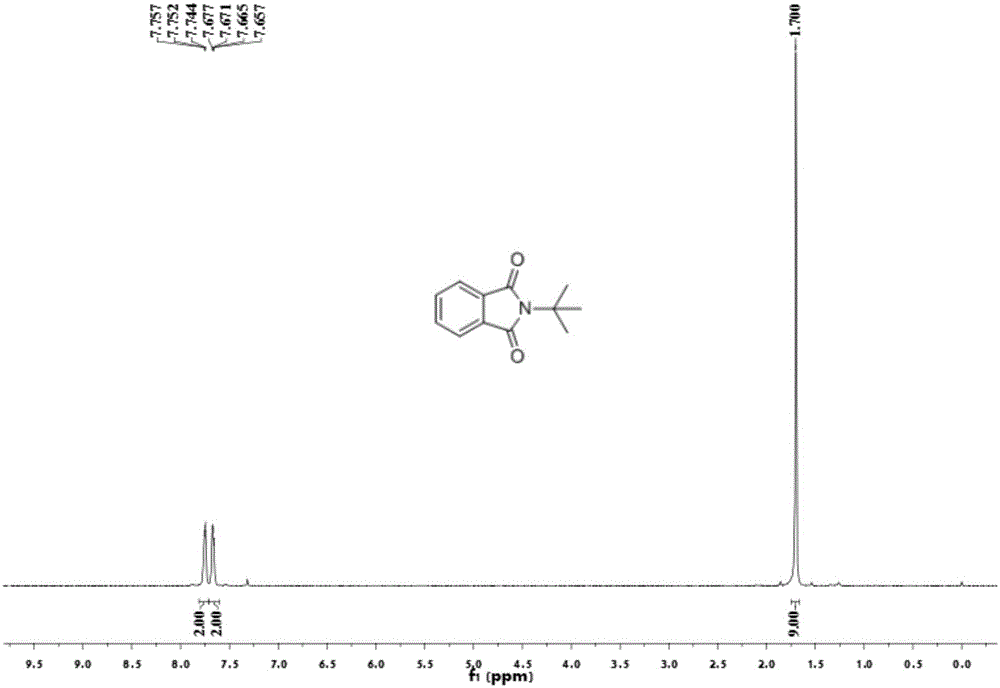
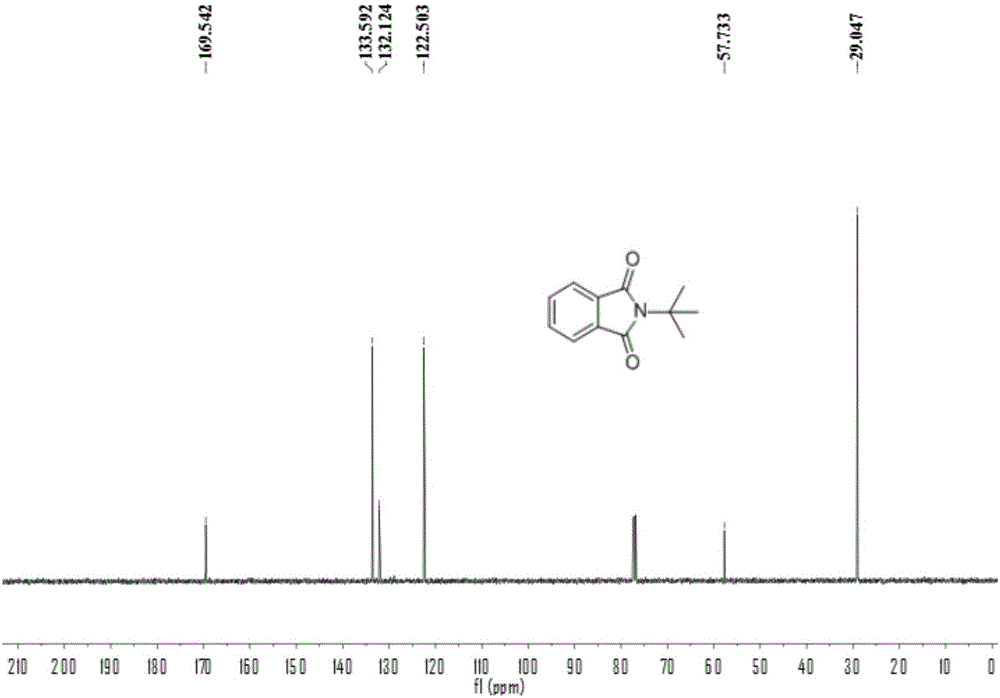
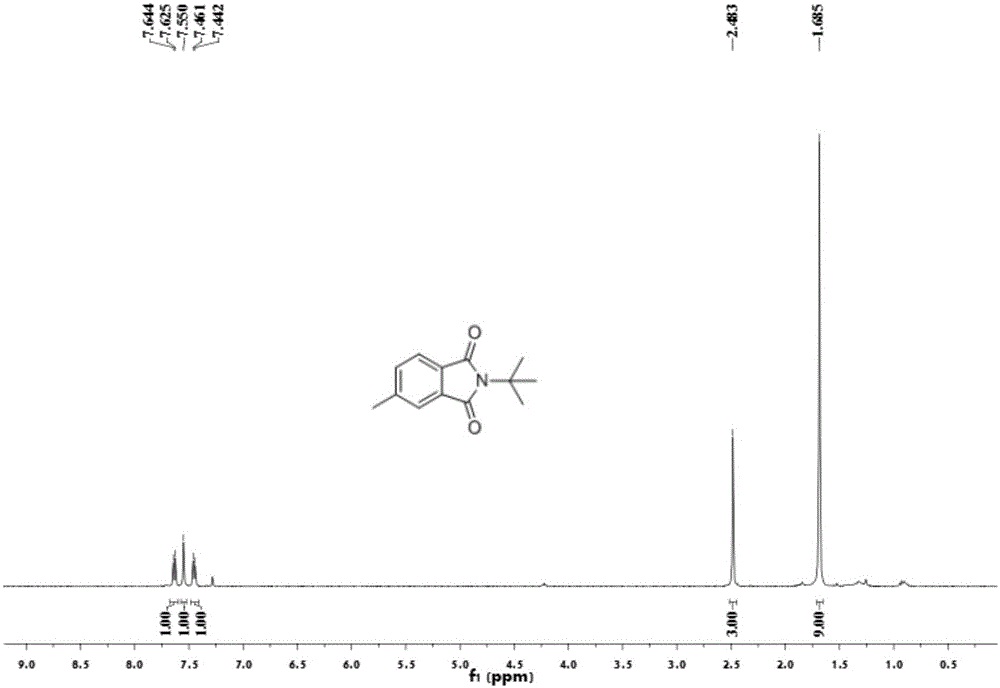
[0054] Add 0.2 mmol of benzaldehyde, 0.4 mmol of tert-butylamine, 0.01 mmol of palladium chloride, 0.2 mmol of copper oxide into a 25 mL test tube, add 3 ml of toluene (PhMe) and N,N-dimethylformamide (DMF) As a mixed solvent, the volume ratio was 10:1, and stirred at room temperature for 12 hours. Then put on a balloon containing carbon monoxide and oxygen as a carbonyl source, and stir at 100 degrees Celsius. After the TLC (thin layer chromatography) detection reaction, the reaction solution was cooled to room temperature, the balloon was removed, and the unreacted carbon monoxide and oxygen were slowly vented. The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate 55%.
Embodiment 2
[0056] Add 0.2 mmol of benzaldehyde, 0.4 mmol of tert-butylamine, 0.01 mmol of bistriphenylphosphine palladium dichloride, 0.2 mmol of copper oxide into a 25 mL test tube, add toluene (PhMe) and N,N-dimethylformaldehyde Amide (DMF) 3 ml was used as a mixed solvent with a volume ratio of 10:1, and stirred at room temperature for 12 hours. Then put on a balloon containing carbon monoxide and oxygen as a carbonyl source, and stir at 100 degrees Celsius. After the TLC (thin layer chromatography) detection reaction, the reaction solution was cooled to room temperature, the balloon was removed, and the unreacted carbon monoxide and oxygen were slowly vented. The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate 21%.
Embodiment 3
[0058] Add 0.2 mmoles of benzaldehyde, 0.4 mmoles of tert-butylamine, 0.01 mmoles of palladium dichlorodiacetonitrile, 0.2 mmoles of copper oxide into a 25 mL test tube, add toluene (PhMe) and N,N-dimethylformamide (DMF) As a mixed solvent, the volume ratio was 10:1, and stirred at room temperature for 12 hours. Then put on a balloon containing carbon monoxide and oxygen as a carbonyl source, and stir at 100 degrees Celsius. After the TLC (thin layer chromatography) detection reaction, the reaction solution was cooled to room temperature, the balloon was removed, and the unreacted carbon monoxide and oxygen were slowly vented. The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate of 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com