Application of isoflavonoids compound to prepare agricultural bactericide
A technology for agricultural fungicides and compounds, which is applied in the field of preparing agricultural fungicides to achieve the effects of excellent bacteriostatic effect, environmental safety and wide application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
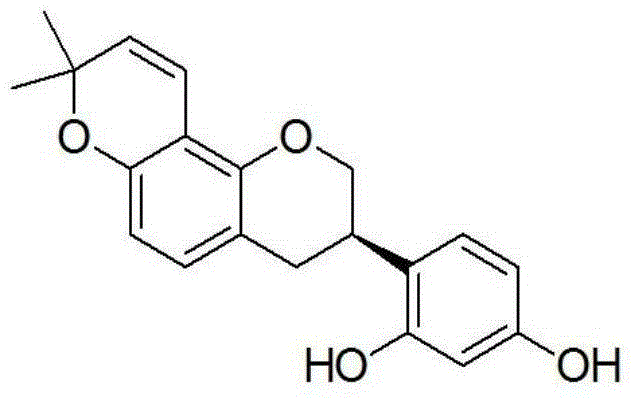
[0023] Antibacterial activity of glabridin of formula (I) of the present invention
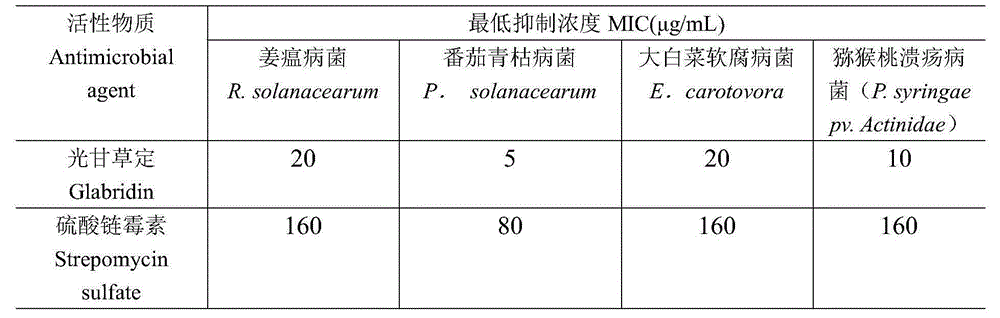
[0024] Microdilution method was used to determine the effect of glabridin on R. solanacearum, R. solanacearum, E. carotovora and P. syringa epv. Actinidae 4 Antibacterial activity of the tested bacteria. All strains were provided by the Pollution-free Pesticide Research Laboratory of Sichuan Agricultural University.
[0025] Insert the bacteria to be tested into Mueller-Hinton broth medium, cultivate at 35°C for 48h, transfer to 0.9% normal saline after being slightly turbid, and adjust to a McFarland turbidity of 0.5 (the measurement method is to use a spectrophotometer to make it The absorbance at 650nm wavelength is 0.020), and then diluted 200 times with Mueller-Hinton broth medium for later use. Connect the diluted bacterial solution to a 96-well plate, add 100 μl of liquid to each well, and then add 100 μl of the drug solution to be tested at different concentrations prepared by steril...
Embodiment 2
[0030] The spore germination inhibition method was used to determine the effect of glabridin on Botrytis cinerea, Fusarium graminearum, Phytophthora infestans, Pyricularia grisea and Colletotrichum gloeosporioides, etc. Antibacterial activity of the tested fungi. All strains were provided by the Pollution-free Pesticide Research Laboratory of Sichuan Agricultural University.
[0031] Inhibition of spore germination method: the spores of pathogenic fungi are made into a spore suspension of a certain concentration (under a 10×10 low-magnification lens, there are 20 to 40 spores per field of view), and different concentrations of glabridin are mixed with the spore suspension. After mixing, the droplets were added onto the concave glass slide, and each treatment was repeated 3 times. Carbendazim was used as the drug control, and sterile water was used as the blank control. After cultivating in moisture at 25°C for 8h to 12h, check the germination of the control spores. After the...
Embodiment 3
[0036] The combined effect of embodiment triglabridin and levoshikonin on pathogenic fungi
[0037] 1.1 Inhibitory toxicity of glabridin and levoshikonin to pathogenic fungi
[0038] The toxicity of glabridin and levoshikonin to wheat scab and tea anthracnose was measured with reference to the method in Example 2.
[0039] 1.2 Research on compounding effect of two compounds
[0040] Composite glabridin and levoshikonin: the two compounds are formulated into 5 ratios (mass ratio) of 3:1, 2:1, 1:1, 1:2, and 1:3 according to the mass ratio . The above formulations were tested for toxicity according to the drug toxicity test method.
[0041] To evaluate the combined effect of the mixture, the EC 50 Based on the value, the joint effect type of the mixture was evaluated by calculating the co-toxicity coefficient of the mixture.
[0042]
[0043] CTC≥120 is a synergistic effect, 80≤CTC<120 is an additive effect, and CTC<80 is an antagonistic effect.
[0044] 1.3 Test results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com