Application of a kind of isoflavone compound for preparing agricultural fungicide
A technology for agricultural fungicides and compounds is applied in the field of preparing agricultural fungicides to achieve the effects of excellent bacteriostatic effect, wide application prospects and environmental safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
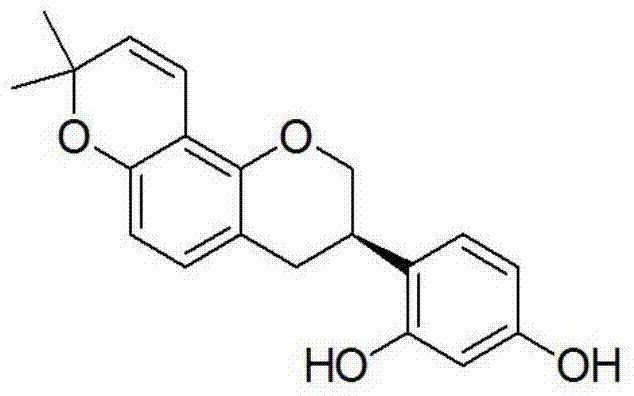
[0023] Antibacterial activity of Glycyrrhizin of formula (I) of the present invention
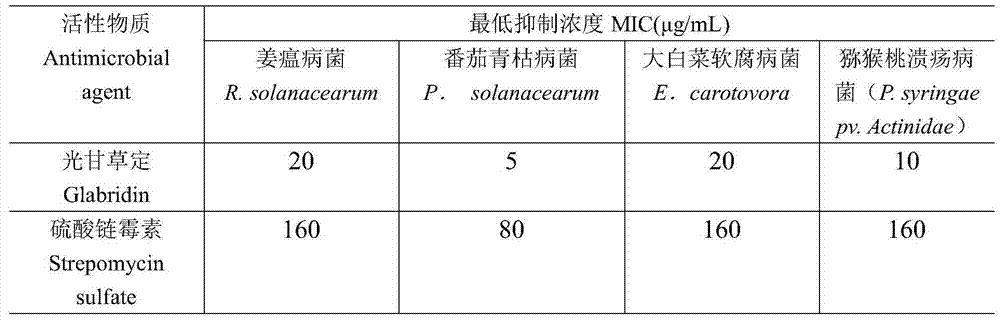
[0024] The microdilution method was used to determine the effects of Glycyrrhizin on ginger blast fungus (R.solanacearum), tomato bacterial wilt (P.solanacearum), cabbage soft rot fungus (E.carotovora), and kiwi fruit canker (P.syringaepv. The antibacterial activity of the tested bacteria. The strains are provided by the Harmless Pesticide Research Laboratory of Sichuan Agricultural University.
[0025] The test bacteria were connected to Mueller-Hinton broth medium and cultured at 35°C for 48 hours. After reaching a slight turbidity, they were transferred to 0.9% saline and adjusted to Mai's turbidity 0.5 (the measurement method is to use a spectrophotometer to make it The absorbance is 0.020 at a wavelength of 650nm), and then diluted 200 times with Mueller-Hinton broth medium for use. Connect the diluted bacterial solution to a 96-well plate, add 100μl to each well, and then add 100μl of th...
Embodiment 2
[0030] The effects of Glycyrrhizin on Botrytis cinerea, Fusarium graminearum, Phytophthora infestans, Pyriculariagrisea, and Colletotrichum (Colletotrichum) gloeosporioides) and other 5 test fungi. The strains are provided by the Harmless Pesticide Research Laboratory of Sichuan Agricultural University.
[0031] Inhibition of spore germination: the spores of pathogenic fungi are mixed into a certain concentration of spore suspension (20-40 spores in each field of view under a 10×10 low power lens), and different concentrations of glycyrrhizin are combined with the spore suspension After mixing, drop the drops on the concave glass slide, and repeat the treatment 3 times. Carbendazim is used as the control of the drug, and sterile water is used as the blank control. After moisturizing culture at 25℃ for 8h-12h, check the germination of control spores. When the control germination rate reached 90%, check the germination rate of all treatments. The spore germ tube length is greate...
Embodiment 3
[0036] Example 3 Combination of Glycyrrhizin and Levo-Shikonin on Pathogenic Fungi
[0037] 1.1 Inhibitory toxicity of Glycyrrhizin and Levo-Shikonin to pathogenic fungi
[0038] The virulence of Glycyrrhizin and Levo-shikonin to Fusarium graminearum and Colletotrichum gloeosporioides was determined with reference to the method in Example 2.
[0039] 1.2 Study on the compound effect of two compounds
[0040] Combine Glycyrrhizin and Levo-Shikonin: The two compounds are formulated into 5 ratios (mass ratio) according to the mass ratio of 3:1, 2:1, 1:1, 1:2, and 1:3 . The above formulas were tested for toxicity according to the method of drug toxicity determination.
[0041] To evaluate the combined effect of the mixture, with EC 50 Based on the value, the combined effect type of the mixture is evaluated by calculating the co-toxicity coefficient of the mixture.
[0042]
[0043] CTC≥120 is synergistic effect, 80≤CTC <120 is additive, CTC <80 is the antagonistic effect.
[0044] 1.3 Test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com