A method for regulating the microporous structure of polylactic acid membranes by in-situ polymerization of bifunctional monomers
A bifunctional, in-situ polymerization technology, applied in chemical instruments and methods, membrane technology, semi-permeable membrane separation, etc., can solve problems such as insufficient interaction force and achieve good hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
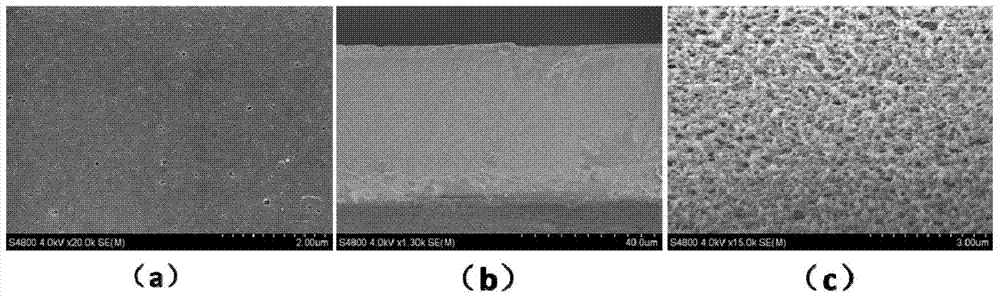
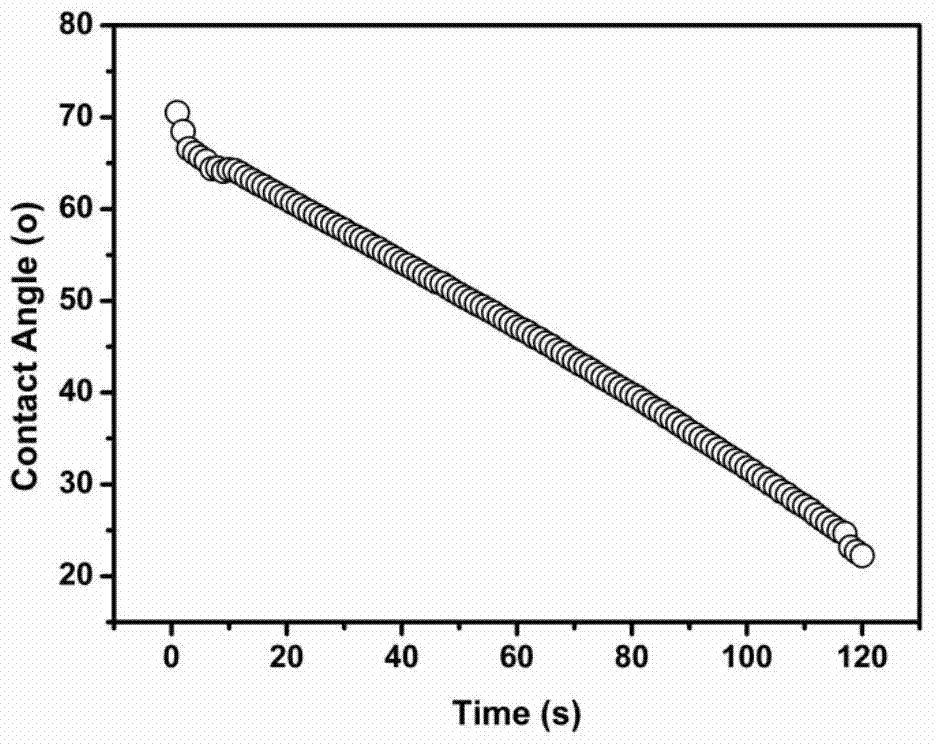
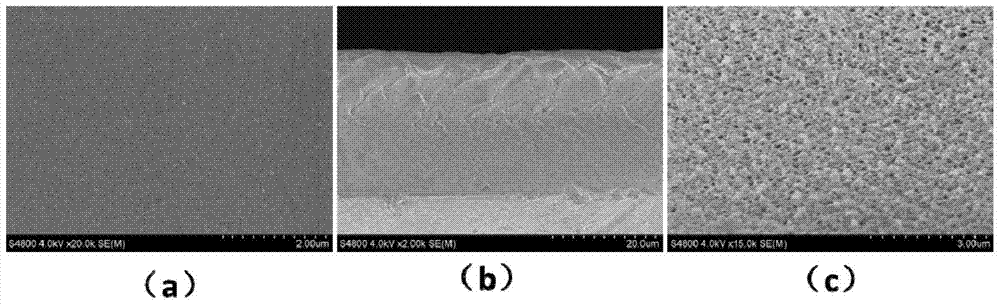
[0025] At a temperature of 80°C and an air humidity of 70%, in a three-necked flask, dissolve 18g of polylactic acid in 82g of N-methylpyrrolidone, and stir the mixture at a stirring speed of 240 rpm for a stirring time of After 12 hours, a film-forming precursor solution containing 18wt% polylactic acid was obtained. Nitrogen was blown into the film-forming precursor solution, 4 grams of glycidyl methacrylate (GMA) was added, and 0.04 grams of azobisisobutyronitrile was added to the flask half an hour later to allow in-situ polymerization to occur. After reacting for 36 hours, the nitrogen gas was cut off and the air was blown in to terminate the reaction. Under the condition of keeping 80° C., the polylactic acid casting solution was obtained by standing still for 8 hours. At a room temperature of 25°C and an indoor humidity of 70%, the casting solution was evenly poured on a glass plate, then immediately transferred to a deionized water coagulation bath at 25°C, and solidi...
Embodiment 2
[0028] At a temperature of 85°C and an air humidity of 70%, in a three-necked flask, dissolve 20 grams of polylactic acid in 80 grams of N-methylpyrrolidone, and stir the mixture at a stirring speed of 440 rpm. For 12 hours, a film-forming precursor solution containing 20wt% polylactic acid was obtained. Nitrogen was blown into the film-forming precursor solution, 8 grams of glycidyl methacrylate (GMA) was added, and 0.08 grams of azobisisobutyronitrile was added to the flask half an hour later to allow in-situ polymerization to occur. After reacting for 24 hours, the nitrogen gas was cut off and the air was blown in to terminate the reaction. Under the condition of maintaining 85° C., the polylactic acid casting solution was obtained by standing still for 8 hours. At a room temperature of 25°C and an indoor humidity of 70%, the casting solution was evenly poured on a glass plate, then immediately transferred to a deionized water coagulation bath at 30°C, and solidified to fo...
Embodiment 3
[0031] At a temperature of 78°C and an air humidity of 70%, in a three-necked flask, dissolve 17 grams of polylactic acid in 83 grams of N-methylpyrrolidone, and stir the mixture at a stirring speed of 240 rpm. For 12 hours, a film-forming precursor solution containing 17wt% polylactic acid was obtained. Nitrogen was blown into the film-forming precursor solution, 6 grams of glycidyl methacrylate (GMA) was added, and 0.06 grams of azobisisobutyronitrile was added to the flask half an hour later to allow in-situ polymerization to occur. After reacting for 30 hours, the nitrogen gas was cut off and the air was blown in to terminate the reaction. Under the condition of keeping 78° C., the polylactic acid casting solution was obtained by standing still for 8 hours. At a room temperature of 25°C and an indoor humidity of 70%, the casting solution was uniformly poured on a glass plate, then immediately transferred to a deionized water coagulation bath at 20°C, and solidified to for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com