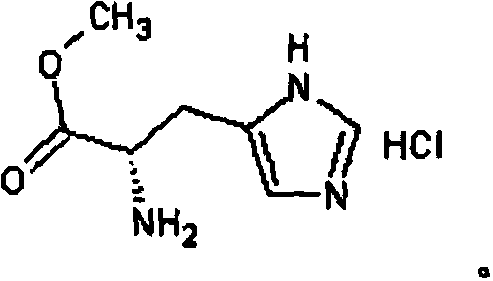
Synthetic method for N-acetyl carnosine
A synthesis method and acetyl carnosine technology, which are applied in the field of N-acetyl carnosine synthesis, can solve the problems of difficult extraction and separation, high price, and difficulty in extraction, separation and purification, and achieve the effects of reasonable process and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
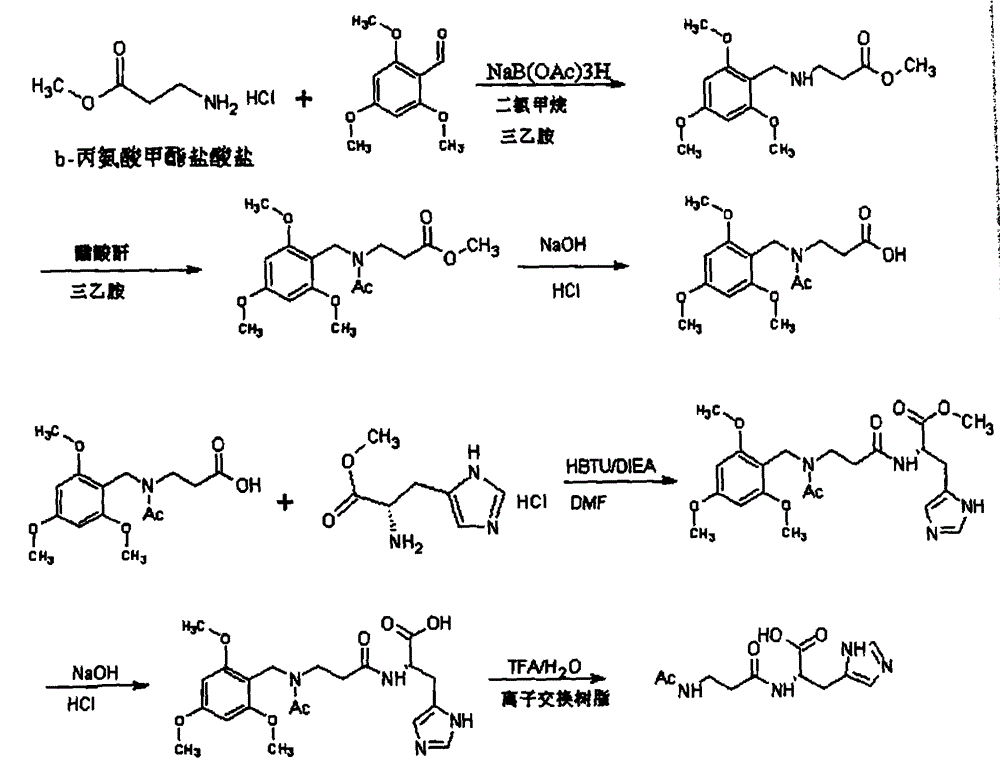
[0024] (1) Synthesis of N-2,4,6-trimethoxybenzyl-β-alanine methyl ester
[0025] Dissolve 10.4 grams of 2,4,6-trimethoxybenzaldehyde in 300 milliliters of dichloromethane, add 11 milliliters of triethylamine, 11.2 grams of β-alanine methyl ester hydrochloride, and 22 grams of sodium acetate borohydride, React at room temperature for 24 hours, add saturated aqueous sodium bicarbonate to separate layers, extract with dichloromethane, wash with water, dry over anhydrous sodium sulfate, filter, and concentrate to obtain N-2,4,6-trimethoxybenzyl-β-alanine methyl Ester oil. The product was directly used in the next reaction without treatment.
[0026] (2) Synthesis of N-acetyl N-2,4,6-trimethoxybenzyl-β-alanine methyl ester
example 1
[0027] The product in Example 1 was dissolved in 100ML of dichloromethane, 14 milliliters of triethylamine was added under stirring, under ice-water bath, 12 milliliters of acetic anhydride was added dropwise, and the temperature was controlled below 10 degrees. Add water, adjust the pH to 1-2 with hydrochloric acid, separate the layers, extract with dichloromethane, wash with saturated brine, dry over anhydrous sodium sulfate, and decolorize. Spin-dry to obtain 17 grams of oil.
[0028] (3) Synthesis of N-acetyl N-2,4,6-trimethoxybenzyl-β-alanine
[0029] Dissolve 8.9 g of N-acetyl N-2,4,6-trimethoxybenzyl-β-alanine methyl ester in 50 ml of methanol, add 20 ml of 4N sodium hydroxide aqueous solution, react for 2 hours, and then adjust the pH with 6N HCl After stirring at 4, a solid precipitated out to obtain a solid, which was washed with ether and dried to obtain 7 g of a solid.
[0030] (4) Synthesis of N-acetyl N-2,4,6-trimethoxybenzyl-β-alanyl-L-histidine
[0031] Add ...
Embodiment 2
[0037] A kind of synthetic method of N-acetylcarnosine, this method comprises the following steps:
[0038](1) Synthesis of N-2,4,6-trimethoxybenzyl-β-alanine methyl ester: using β-alanine methyl ester as starting material, 2,4,6-trimethoxybenzene Formaldehyde and β-alanine methyl ester are reduced by sodium acetate borohydride to obtain N-2,4,6-trimethoxybenzyl-β-alanine methyl ester; the 2,4,6-trimethoxy The mass ratio of benzaldehyde, β-alanine methyl ester, and sodium acetate borohydride is in the range of 1:1:2;
[0039] The 2,4,6-trimethoxybenzaldehyde is dissolved in an organic solvent, and then reacted with β-alanine methyl ester and sodium acetate borohydride. The reaction temperature is room temperature, and the reaction time is 24 hours. The organic solvent is a mixture of dichloromethane and triethylamine in a volume ratio of 300:11.
[0040] (2) Synthesis of N-acetyl N-2,4,6-trimethoxybenzyl-β-alanine methyl ester: N-2,4,6-trimethoxybenzyl-β-alanine obtained in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com