Antistatic agent, molded article comprising insulator polymer material, and method for producing same
A technology of polymer materials and antistatic agents, applied in the field of antistatic agents, can solve the problems of insufficient, inability to obtain antistatic effects, lack of donor/acceptor molecular compounds, etc., and achieve good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0058] Hereinafter, although an Example and a comparative example are shown and this invention is demonstrated more concretely, this invention is not limited to these Examples. In addition, "parts" and "%" in an example are "parts by mass" and "% by mass" unless otherwise specified.
[0059]
[0060] Add 2 moles of glycerin and 1 mole of boric acid to a four-necked flask equipped with a stirrer, a thermometer, a nitrogen inflow pipe and a water detection pipe attached to a condenser installed in the heater, and heat and stir under nitrogen flow to 210 °C under normal pressure. °C, complete dehydration of 3 mole parts. Next, 2 moles of linear stearic acid were added, followed by esterification of fatty acid under normal pressure at 100-250° C. under nitrogen flow, and dehydration of 2 moles was completed to complete the reaction.
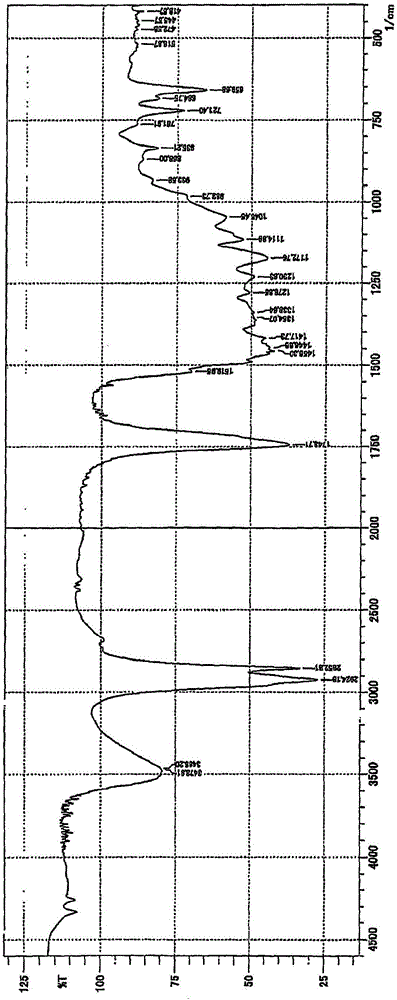
[0061] Next, in the IR absorption spectrum analysis, at 830 ~ 835cm -1 After confirming the generation of the semi-polar organoboron compound of...
Embodiment 2
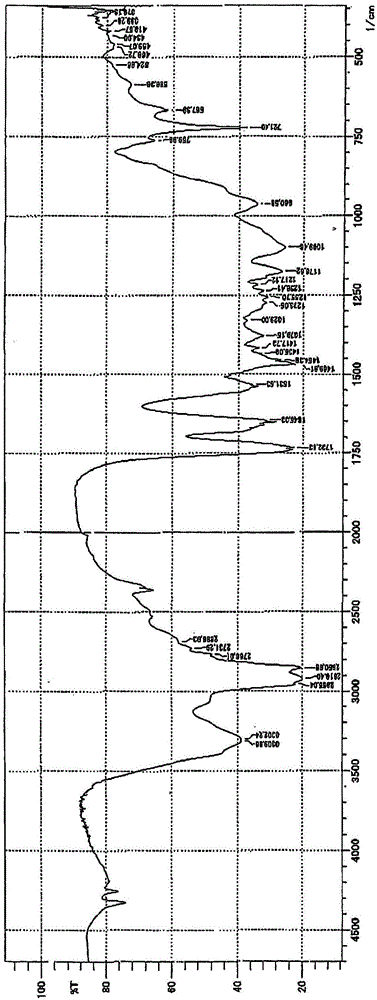
[0079]Add 2 moles of glycerin and 1 mole of boric acid into the same four-neck flask as in Example 1, heat and stir to 210° C. to complete dehydration of 3 moles. Next, 1 mole of linear methyl stearate was added, transesterification was carried out at 230-240° C., and 1 mole of methanol was distilled out of the system to obtain a semipolar organoboron compound as a donor component. The confirmation of the generation of this boron compound was carried out by IR absorption spectrum analysis similarly to Example 1. In addition, the structural displacement acid value of this boron compound measured in the same manner as in Example 1 was 122.2 (theoretical value 122.3).
[0080] In addition, use the same four-necked flask as above to carry out the amidation reaction of 1 mole of diethylaminopropylamine and 1 mole of linear stearic acid, and complete the dehydration corresponding to 1 mole equivalent to obtain the tertiary amine of the acceptor component . The amine value of this ...
Embodiment 3
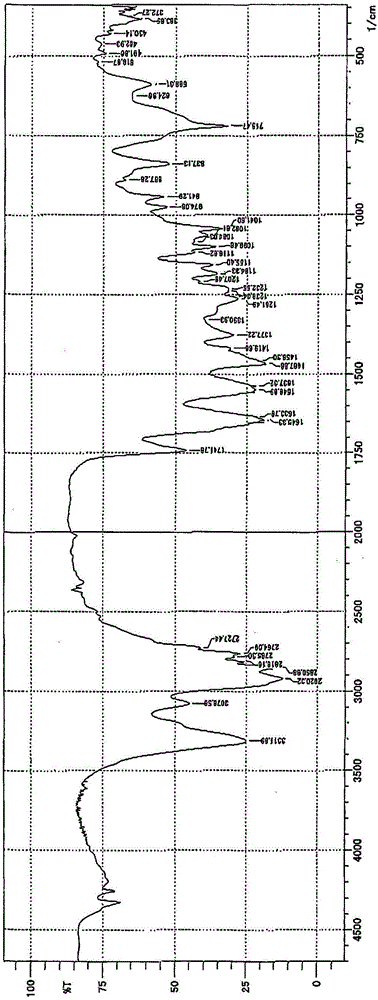
[0085] Add 2 moles of glyceryl monostearate and 1 mole of triethyl borate into the same four-necked flask as in Example 1, carry out transesterification reaction at 100-200°C, and distill 3 moles of ethanol To the outside of the system, a semipolar organic boron compound as a donor component is obtained. The confirmation of the generation of this boron compound was carried out by IR absorption spectrum analysis similarly to Example 1. In addition, the structural shift acid value of this boron compound measured in the same manner as in Example 1 was 77.5 (theoretical value 77.4).
[0086] In addition, 2 moles of ethylene oxide were reacted with 1 mole of stearic acid monoamide of ethylenediamine without a catalyst to obtain a tertiary amine as an acceptor component. The amine value of the obtained tertiary amine measured in the same manner as in Example 1 was 135.2 (theoretical value 135.3).
[0087] Next, 1 mole of the tertiary amine was added to 1 mole of the semi-polar org...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com