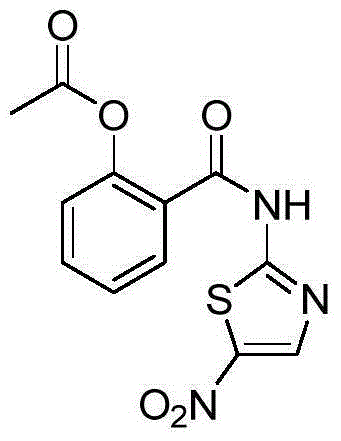
Preparation method of nitazoxanide
A technology of nitazoxanide and nitrothiazole, which is applied in the field of preparation of nitazoxanide, can solve the problems of low solubility, large environmental pollution, and high cost, and achieve the effects of high yield, high product purity, and short cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
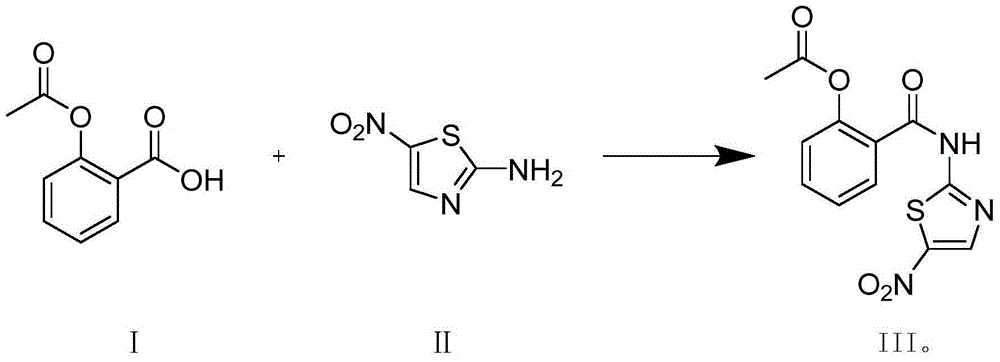
[0019] A preparation method of nitazoxanide according to the present invention, the preparation method is: using acetylsalicylic acid represented by formula (I) as raw material, and 2-amino-5 -Nitrothiazole reacts in the presence of a condensation agent and a catalyst, the reaction mixture is cooled to room temperature, filtered, pickled, dried, concentrated, refined to obtain the compound shown in the corresponding formula (III);
[0020]
[0021] The condensing agent described in the present invention is selected from N,N'-carbonyldiimidazole (CDI), 2-(7-aza-1H-benzotriazol-1-yl)-1,1,3,3- One of tetramethyluronium hexafluorophosphate (HATU), benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU);
[0022] The catalyst is selected from one of 4-dimethylaminopyridine (DMAP), 1-hydroxybenzotriazole (HOBt), N-hydroxy-7-azabenzotriazole (HOAt);
[0023] In the reaction process, a reaction solvent A is also added, and the reaction solvent A is selected from N,N-...
Embodiment 1
[0026] Add 20g of acetylsalicylic acid into 200ml of anhydrous tetrahydrofuran, add 1.0g of DMAP, add 29g of CDI in batches at room temperature, stir for 2-3 hours, add 13g of 2-amino-5-nitrothiazole and 100ml of tetrahydrofuran dropwise at 0-5°C After the dropwise addition, raise the temperature and reflux to react for 24 hours, cool to room temperature, filter, add saturated sodium bicarbonate 100ml to the filter cake and stir for 20 minutes, 100ml of water and stir for 20 minutes, rinse with ethanol to obtain the crude product, then use DMF and methanol Recrystallization gave 11.3 g of nitazoxanide.
Embodiment 2
[0028] Add 20g of acetylsalicylic acid into 200ml of anhydrous DMF, 3g of HOBt, add 14g of 2-amino-5-nitrothiazole, cool to 0°C, add 17g of HATU in batches, heat up to room temperature and react for 10 hours, concentrate, add dichloromethane and stir , filtered to obtain the crude product, then the crude product was added to 100ml of 1mol / L hydrochloric acid and stirred for 20 minutes, filtered, stirred in 100ml of saturated sodium bicarbonate for 20 minutes, recrystallized from DMF and methanol to obtain 8.75g of nitazoxanide. Yield 36%.
[0029] Structure analysis
[0030] 1. UV absorption spectrum
[0031] Instrument: HITACHIU-3010 UV Analyzer
[0032] Solvent: ethanol, 0.1mol / L hydrochloric acid, 0.1mol / L sodium hydroxide
[0033] 1) Measurement data
[0034] The ultraviolet-visible spectral data of table 1 sample
[0035]
[0036] 2) Analysis
[0037] The maximum UV absorption of the sample in ethanol solution is λ max =204nm, ε=2.17×10 4 , for the unsaturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com