Arsenic sugar compound with anti-tumor activity and preparation method therefor and application thereof
A synthesis method and reaction technology, applied to arsenic sugar compound with anti-tumor activity and its preparation method and application field, capable of solving the problems of high cytotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 4-(1,3,2-disulfarsane)aniline, using methanol as solvent. Through the first method, 4-dichloroarsenaniline hydrochloride is first prepared, and then hydrolyzed under the action of ammonia water to obtain 4-aminophenyl arsenic oxide (3-valent arsenic), and then reacted with 1,2-ethanedithiol to prepare 4-(1 ,3,2-Disulfurarsane) aniline. Weigh arsanic acid (p-aminobenzene arsenic acid) (10g, 46.08mmol), potassium iodide (50mg, 0.30) and dissolve in 60mL methanol and 48mL concentrated hydrochloric acid mixed solvent. Introduce sulfur dioxide gas for 30 minutes until the solution turns from orange red to light yellow. A white solid precipitated out. The solution cools down to 0 o C, let stand overnight. Suction filtration, the filter cake was washed twice with 50 mL of cold diethyl ether. 4-Dichloroarsenaniline hydrochloride (12.57 g, 95%) was obtained as a white solid.
[0026] 4-Dichloroarsenaniline hydrochloride (12.57g, 43.78mmol) was add...
Embodiment 2
[0028] Example 2: Preparation of 4-(1,3,2-disulfarsane)aniline, using methanol as solvent. 4-dichloroarsenaniline hydrochloride is firstly prepared by method 2, and 4-(1,3,2-disulfurarsenane) aniline is prepared by directly reacting with 1,2-ethanedithiol.
[0029] Weigh arsanic acid (p-aminobenzene arsenic acid) (10g, 46.08mmol), potassium iodide (50mg, 0.30) and dissolve in 60mL methanol and 48mL concentrated hydrochloric acid mixed solvent. Introduce sulfur dioxide gas for 30 minutes until the solution turns from orange red to light yellow. A white solid precipitated out. The solution cools down to 0 o C, let stand overnight. Suction filtration, the filter cake was washed twice with 50 mL of cold diethyl ether. 4-Dichloroarsenaniline hydrochloride (12.57 g, 95%) was obtained as a white solid.
[0030] 4-Dichloroarsenaniline hydrochloride (12.57g, 57.96mmol) was dissolved in 300mL aqueous solution, cooled to 0-5 o C, adding sodium carbonate (total 10.65 g, 57.96 mmol) ...
Embodiment 3
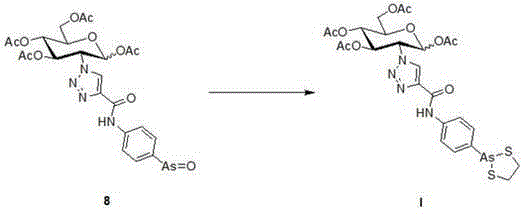
[0031] Example 3: Using 4-(1,3,2-disulfarsane)aniline as raw material and dichloromethane as solvent. It can generate N-(4-(1,3,2-disulfarsane)phenyl)alkynamide with propargyl acid under the action of N,N'-cyclohexylcarbodiimide.
[0032] Add propargyl acid (216uL, 3.521mmol) into dichloromethane (10mL) dissolved in N,N'-cyclohexylcarbodiimide (726.5mg, 3.521mmol), stir at room temperature for 1 hour, cool down in an ice bath and stir After 30 min (1,3,2-disulfarsane)aniline (760.6 mg, 2.934 mmol) in dichloromethane (10 mL) was added dropwise. After the dropwise addition was completed, the reaction was incubated for 30 min, and the temperature was gradually raised to room temperature for overnight reaction. The reaction is complete. Celite was filtered, the solvent was evaporated to dryness under reduced pressure, and the residue was subjected to column chromatography (eluent: petroleum ether: ethyl acetate = 4:1) to obtain a yellow solid (584.5mg, 64%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com