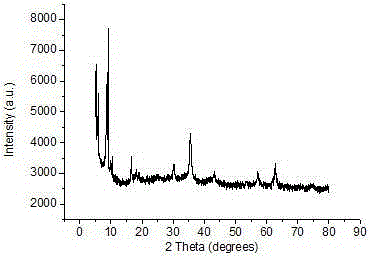
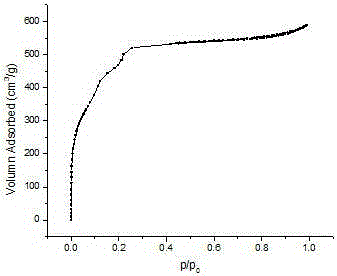
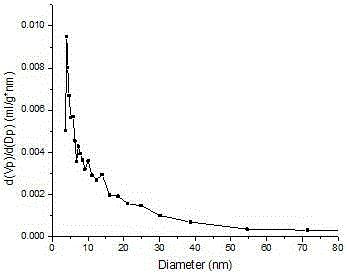
Preparation method and application of Fe3O4/MIL-101(Cr)
A 3.9H2O, NH3.H2O technology, applied in chemical instruments and methods, adsorption water/sewage treatment, alkali metal oxides/hydroxides, etc., can solve the problem of large particle size of magnetic nanoparticles and reduced surface area of composite materials , Magnetic weakening and other problems, to achieve the effect of stable quality, large surface area and strong magnetism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) MIL-101(Cr) was synthesized by hydrothermal method. Weigh 800mgCr(NO 3 ) 3 9H 2 O and 332mg of terephthalic acid, placed in a polytetrafluoroethylene liner, add 0.4mL of hydrofluoric acid with a mass fraction of 35% and 9.5mL of pure water, ultrasonic for 15min, after mixing evenly, cover and move to a stainless steel reaction kettle After inner sealing, place in an oven at 215° C., react for 9 hours, and then cool down to room temperature naturally. The product is a green powder, which is washed three times with N,N-dimethylformamide, ethanol and pure water respectively, centrifuged at high speed and dried for use.
[0032] (2) Preparation of Fe by reduction precipitation method 3 o 4 / MIL-101 (Cr) composite material. Weigh 0.125g of MIL-101(Cr), place it in a 100mL three-necked flask, add 60mL of 0.05mol / L FeCl 3 After the solution was shaken and mixed evenly, it was sonicated at room temperature 25°C for 30 minutes, and then nitrogen gas was passed into th...
Embodiment 2
[0035] (1) MIL-101(Cr) was synthesized by hydrothermal method. Weigh 800mgCr(NO 3 ) 3 9H 2 O and 332mg of terephthalic acid, placed in a polytetrafluoroethylene liner, add 0.4mL of hydrofluoric acid with a mass fraction of 35% and 9.5mL of pure water, ultrasonic for 15min, after mixing evenly, cover and move to a stainless steel reaction kettle After inner sealing, place in an oven at 220° C., react for 8 hours, and then cool to room temperature naturally. The product is a green powder, which is washed three times with N,N-dimethylformamide, ethanol and pure water respectively, centrifuged at high speed and dried for use.
[0036] (2) Preparation of Fe by reduction precipitation method 3 o 4 / MIL-101 (Cr) composite material. Weigh 0.500g MIL-101(Cr), place it in a 100mL three-necked flask, add 30mL FeCl with a concentration of 0.1mol / L 3 After the solution was shaken and mixed evenly, it was sonicated at room temperature 25°C for 30 minutes, then nitrogen gas was passed...
Embodiment 3
[0039] (1) MIL-101(Cr) was synthesized by hydrothermal method. Weigh 800mgCr(NO 3 ) 3 9H 2 O and 332mg of terephthalic acid, placed in a polytetrafluoroethylene liner, add 0.35mL of hydrofluoric acid with a mass fraction of 40% and 9.5mL of pure water, ultrasonic for 15min, after mixing evenly, cover and move to a stainless steel reaction kettle After inner sealing, place in an oven at 225° C., react for 7 hours, and then cool to room temperature naturally. The product is a green powder, which is washed three times with N,N-dimethylformamide, ethanol and pure water respectively, centrifuged at high speed and dried for use.
[0040] (2) Preparation of Fe by reduction precipitation method 3 o 4 / MIL-101 (Cr) composite material. Weigh 1.000g of MIL-101(Cr), place it in a 100mL three-necked flask, add 15mL of 0.2mol / LFeCl 3 After the solution was oscillated and mixed evenly, it was sonicated at room temperature 25°C for 30 minutes, then nitrogen gas was passed into the thre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com