VP (viral protein)0 recombinant protein of DHAV (duck hepatitis A virus)-1 as well as preparation method and application of VP0 recombinant protein
A duck hepatitis A virus and recombinant protein technology, applied in the field of biomedicine, can solve the problems of low accuracy of duck viral hepatitis antibody, difficult expression and purification of VP0 protein, low detection efficiency, etc., and achieves good reactogenicity and strong specificity. , good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A type 1 duck hepatitis A virus VPO recombinant protein, its amino acid sequence is shown in SEQ ID NO: 1, and its DNA sequence is shown in SEQ ID NO: 2.
[0054] A preparation method of type 1 duck hepatitis A virus VPO recombinant protein, comprising the steps of:
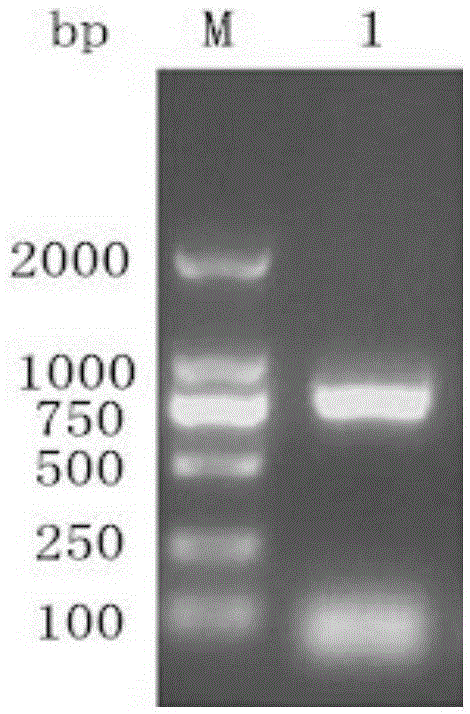
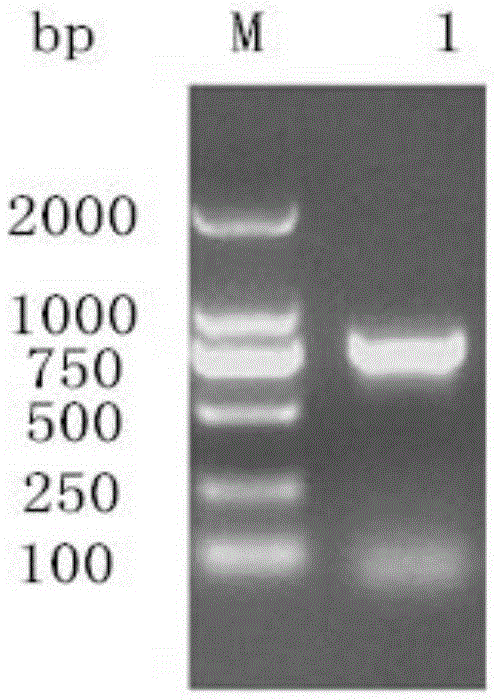
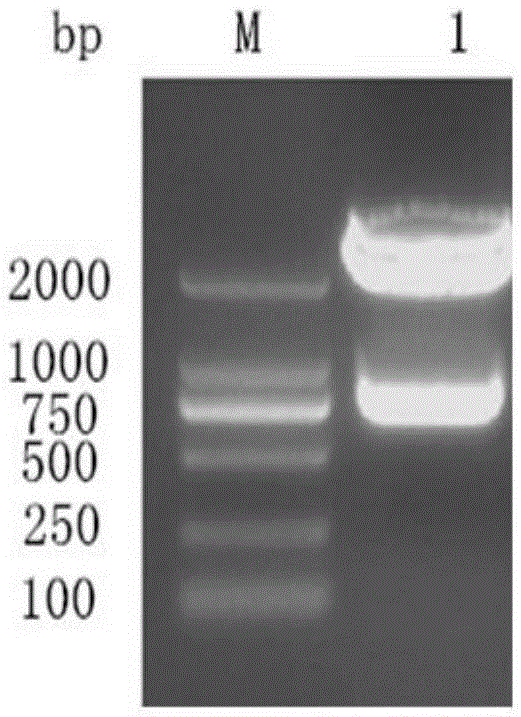
[0055] Step 1: Cloning of the whole gene of duck hepatitis A virus VP0 type 1: analysis and determination of the whole gene of duck hepatitis A virus type 1 VP0 and restriction sites, and design and amplification according to the whole gene sequence of DHAV-1X strain with Genbank accession number JQ316452.1 Specific primers for the DNA fragment of the VPO gene of duck hepatitis A virus type 1. At the same time, two enzyme cutting sites, BamHI and XhoI, were added to the 5' end of the primers to extract the RNA of duck hepatitis A virus type 1, and the specific primers were used for RT-PCR amplification. The target fragment of the whole gene of type 1 duck hepatitis A virus VPO was obtained.
[0056] Where...
Embodiment 2
[0101] The optimization of embodiment 2 recombinant protein expression conditions
[0102] experimental method:
[0103] 1. Optimization of IPTG concentration
[0104] Inoculate the expressing bacteria into four LB / Amp medium test tubes at a ratio of 1:100, and culture at 37°C until OD 600nm At 0.6, add IPTG to the final concentration: 0mmol / L, 0.4mmol / L, 0.8mmol / L and 1.2mmol / L to induce culture for about 4h, sample treatment, SDS-PAGE electrophoresis.
[0105] 2. Optimization of induction time
[0106] Inoculate the expressing bacteria into 5 LB / Amp medium test tubes according to the ratio of 1:100, and wait until OD 600nm After reaching 0.6, add the optimal IPTG concentration, respectively induce 4h, 6h, 8h, 10h and 12h, the treatment is the same as above.
[0107] 3. Optimization of induction temperature
[0108] Inoculate the expressing bacteria into 3 LB / Amp medium test tubes at a ratio of 1:100, and wait until OD 600nm After reaching 0.6, add the optimal IPTG conc...
Embodiment 3
[0111] Embodiment 3 Establishment of ELISA method based on recombinant protein
[0112] experiment method:
[0113] 1. Optimization of reaction conditions for recombinant protein-based ELISA method
[0114] The ELISA reaction conditions are operated according to the conventional method, and the optimization of the ELISA method reaction conditions is as follows:
[0115] (1) Antigen coating concentration: VP0 recombinant protein (1mg / ml) made 1:100, 1:200, 1:300, 1:400, 1:600, 1:800, 1:1200, 1:1600 and 1 : 2400 nine dilutions;
[0116] (2) Optimum dilution of serum: DHAV-1 positive / negative serum is 1:10; 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640 seven dilutions ;
[0117] (3) The optimal dilution of the enzyme-labeled antibody: set five dilutions of 1:200, 1:400, 1:800, 1:1600 and 1:3200;
[0118] (4) Protein coating conditions: 37°C for 1h to 4°C overnight, 37°C for 4h to 4°C overnight, 4°C overnight and 37°C for 2h;
[0119] (5) Selection of blocking solution: set six b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com