Antigen epitope based on il-6 and its application
An antigen epitope and amino acid technology, applied in the field of biomedicine, can solve the problems of high price and long service life of antibody drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] 1. Transplantation of linear B epitopes
[0157] In this example, 6 fusion epitopes were designed, and DTT was used as the carrier to prepare the basis for immune antigen peptides. The transplantation results are summarized in Table 1.
[0158] Table 1 The design of IL-6 epitope transplantation to DTT
[0159]
[0160] 2. Preparation of Antigenic Peptides
[0161] 2.1 Primer design
[0162] According to the human IL-6 domain, murine IL-6 domain, five candidate epitopes designed and the base sequence information of DTT, primers were designed using Primer5.0 software. Among them, the two structural domains are respectively inserted at the carboxy terminus (C terminus) of DTT, and the designed primers are named hP1, hP2, mP1, mP2. The insertion of the epitope into DTT requires the design of four primers, named P1, P2, P3, and P4, respectively, wherein the primers P1, P4 are consistent, and BamHI is inserted at the 5' end and 3' end of the P1, P4 primers respectively ...
Embodiment 2
[0209] Embodiment 2 animal immunity and activity detection
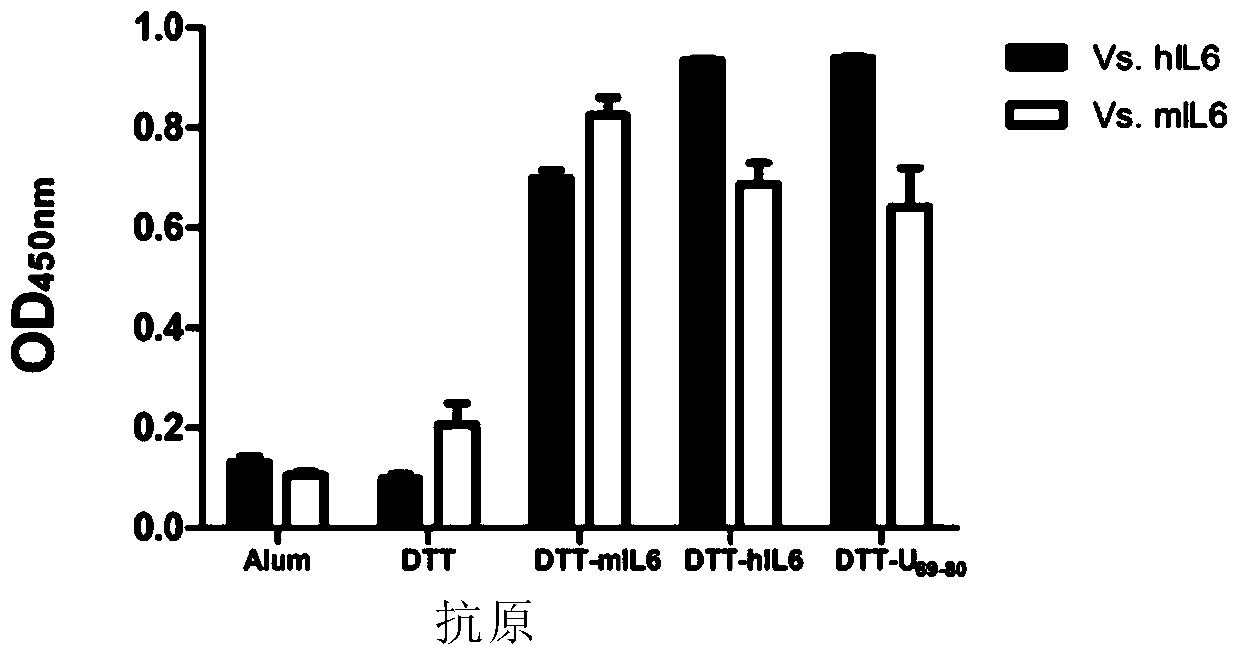
[0210] 1 Animal immunity and determination of antigen-antibody binding strength
[0211] 1.1 Immunization and blood collection of animals
[0212] Sixty BalB / c mice aged 6-8 weeks were randomly divided into 11 groups, aluminum adjuvant control group, empty vector DTT control group, and 8 experimental groups, 6 mice in each group. The antigen was mixed with aluminum hydroxide (final concentration 1.6 mg / mL) adjuvant 1:1, and the injection dose was 50 μg antigen / animal / time, and multi-point subcutaneous injection on the back. Immunization was performed every two weeks after the first immunization, and antiserum was collected one week after the third immunization.
[0213] 1.2 ELISA method to detect the production of antibodies
[0214] In order to determine whether antibodies against hIL6 or mIL6 are produced in the serum of the experimental group, an ELISA experiment is required. The process of ELISA assay antibod...
Embodiment 3
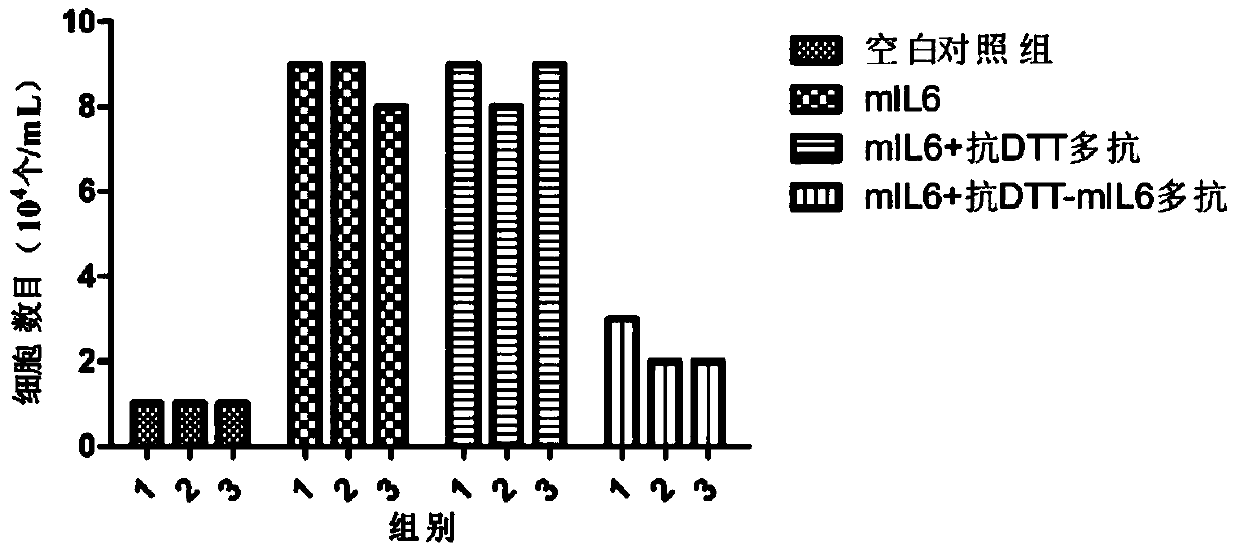
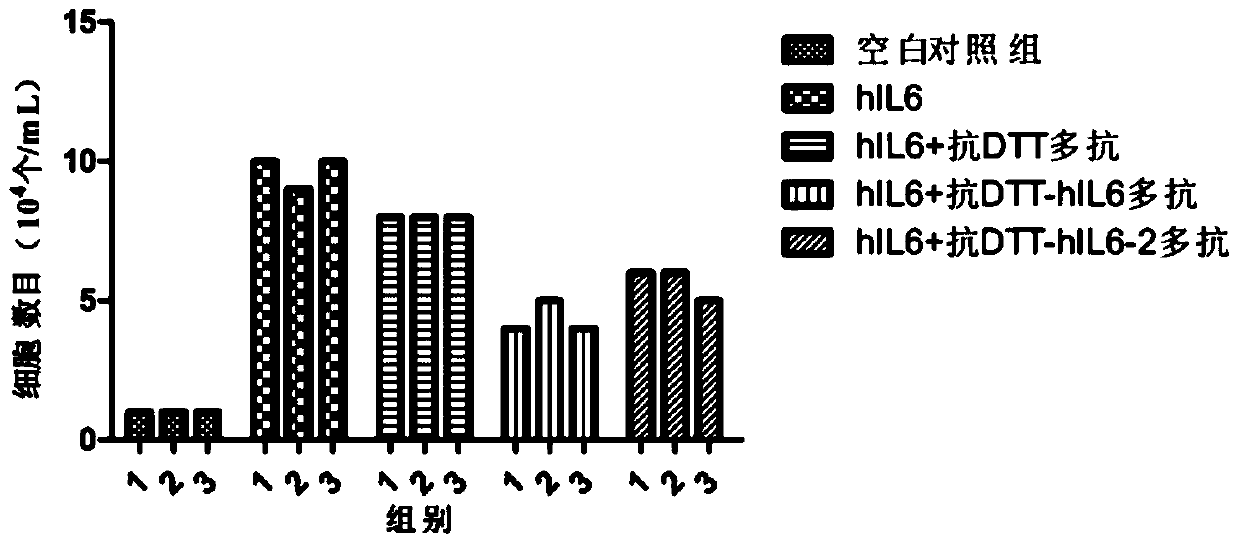
[0237] Example 3 The determination of the activity of neutralizing interleukin-6 by multiple antibodies
[0238] 1. Purification and quantification of polyclonal antibodies
[0239] The polyclonal antibody was purified by conventional methods in the field, and the purity of the polyclonal antibody obtained by electrophoresis analysis was over 90%, and the polyclonal antibody was quantified by BCA method.
[0240] 2. Exploration of the dosage of multiple antibodies to neutralize IL-6 activity in vitro
[0241] 1) Take out 1 tube (about 107 / mL) of 7TD1 cells frozen in liquid nitrogen (purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences) in an ice box, freeze and thaw quickly at 37°C, add 7 mL of RPMI1640 medium and mix well Afterwards, centrifuge to remove the supernatant (wash away DMSO immediately);
[0242] 2) Resuspend 500 μL of cell cryopreservation medium in 7 mL RPMI1640 medium (containing 10% FBS, 128 pg / mL mIL6) and 7 mL RPMI1640 medium (containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com