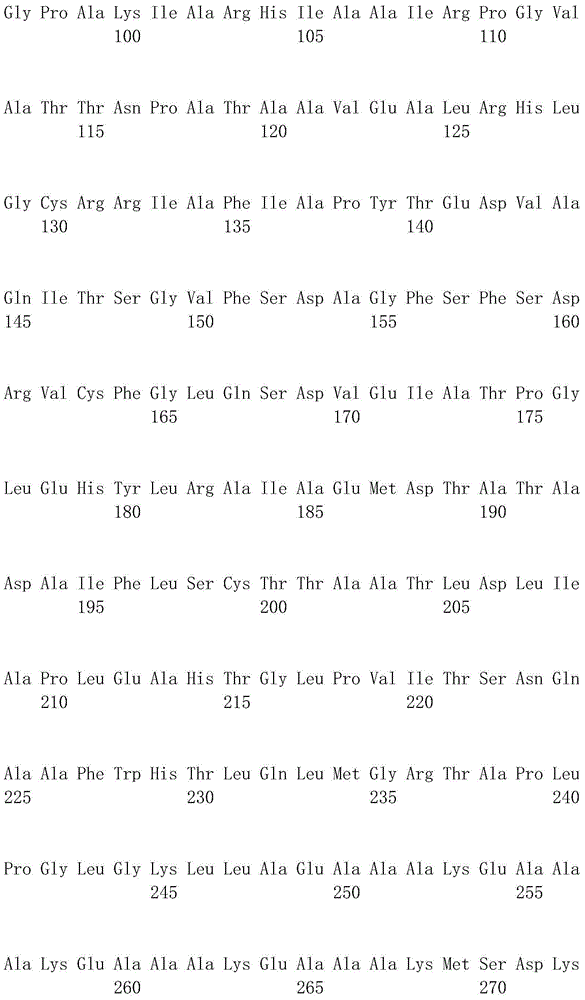Method for synthesizing L-cysteine by transforming DL-2-amino-delta<2>-thiazolinyl-4-carboxylic acid (DL-ATC) by enzyme process
A technology for the conversion of cysteine, enzymatically, in the direction of microbial-based methods, biochemical devices and methods, immobilized on/in organic carriers, etc., capable of addressing variability, short half-life, wild pseudomonas The difficulty of bacterial transformation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Knockout of Escherichia coli JM109 (DE3) L-cysteine desulfhydrylase gene tnaA
[0058] 1. Materials
[0059] Strains: Escherichia coli JM109 (DE3), purchased from Promega.
[0060] Plasmids: Plasmids pKD4, pKD46, and pCP20 were purchased from Wuhan Miaoling Biotechnology Co., Ltd.
[0061] LB medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L.
[0062] Kanamycin-resistant plate: LB solid medium containing 20 mg / L kanamycin and 1.5% agar powder.
[0063] SOC medium: peptone 2g / L, yeast powder 0.5g / L, NaCl0.0585g / L, KCl0.0186g / L, MgCl 2 0.203g, MgSO 4 0.246g / L, glucose 20mmol / L.
[0064] 2. Method
[0065] (1) Cloning of tnaA gene homologous recombination fragment
[0066] The target gene was knocked out using the Red recombination system. Primers were designed according to the sequence of the Escherichia coli tnaA gene (Genebank accession number: K00032.1) published by Genbank:
[0067] tnaAup:
[0068] 5'-ATGGAAAACTTTAAACATCTCCCTGAACCGTTCCGCATTCG...
Embodiment 2
[0088] Example 2 Knockout of Escherichia coli JM109 (DE3) ΔtnaAL-cysteine desulfhydrylase gene malY
[0089] 1. Materials
[0090] Bacterial species: Escherichia coli engineering strain JM109(DE3)ΔtnaA, see Example 1 for its related characteristics.
[0091] Plasmids: Plasmids pKD4, pKD46, and pCP20 were purchased from Wuhan Miaoling Biotechnology Co., Ltd.
[0092] LB medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L.
[0093] Kanamycin-resistant plate: LB solid medium containing 20 mg / L kanamycin and 1.5% agar powder.
[0094] SOC medium: peptone 2g / L, yeast powder 0.5g / L, NaCl0.0585g / L, KCl0.0186g / L, MgCl 2 0.203g, MgSO 4 0.246g / L, glucose 20mmol / L.
[0095] 2. Method
[0096] (1) Cloning of malY gene homologous recombination fragment
[0097] The target gene was knocked out using the Red recombination system. Primers were designed according to the sequence of the Escherichia coli malY gene (Genbank accession number: M60722.1) published by Genbank:
[0098] ma...
Embodiment 3
[0119] Example 3 Conversion of DL-ATC to the construction of a metabolic pathway for synthesizing L-cysteine
[0120] 1. Materials
[0121] Bacterial species: Escherichia coli engineering strain JM109(DE3)ΔtnaAΔmalY, see Example 2 for its related characteristics.
[0122] Plasmid: Plasmid pET-28a(+) was purchased from Novagen, and plasmid pACYC184 was purchased from NewEngland Biolabs.
[0123] LB medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L.
[0124] DL-ATC racemase gene atcA, L-ATC hydrolase gene atcB, nitrogen-carbamoyl-L-cysteine hydrolase gene atcC are all derived from Pseudomonas sp.BS strain.
[0125] Kanamycin+tetracycline double-antibody plate: LB solid medium containing 1.5% agar, 20mg / L kanamycin, and 10mg / L tetracycline.
[0126] 2. Method
[0127] (1) Construction of atcAB expression vector
[0128]The DL-ATC racemase gene (atcA) (GenBank accession number: BAD15357) and the L-ATC hydrolase gene (atcB) derived from the genome of Pseudomonas sp.BS str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com