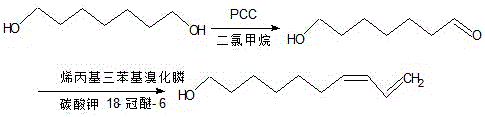
The synthetic method of z7,9-decadiene-1-alcohol
A synthesis method and decadiene technology are applied in the field of chemical synthesis of insect sex pheromones, can solve the problems of difficult products in the synthesis method, separation of intermediate products, long synthesis routes, etc., and achieve low cost, short reaction time, and less three wastes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] (1) Synthesis of 7-hydroxyl-1-heptanal
[0015] Add ground PCC (9.5g, 44.2mmol),
[0016] Silica gel 9.5 g in 95 mL of anhydrous carbon dichloride. Under nitrogen protection, 1,7-heptanediol (5.80g, 43.9mmol) was quickly
[0017] Add quickly and keep stirring, react at room temperature for 3h, then add 19mL of distilled water and then add 95mL of diethyl ether for extraction, and then stir
[0018] 30min, then use silica gel suction filtration, transfer the filtrate to a separatory funnel, add dropwise 20% sodium hydroxide to make the filtrate neutral, extract with ether
[0019] Take three times and combine the organic phases, wash three times with saturated sodium chloride, dry over anhydrous sodium sulfate for 12 hours, evaporate the solvent, and separate the residue with silica gel column to obtain 4.25 g of 7-hydroxy-1-heptanal, with a yield of 73.3%.
[0020] (2) Synthesis of Z7,9-decadien-1-ol
[0021] Add allyl triphenyl
Embodiment 2
[0024] (1) Synthesis of 7-hydroxyl-1-heptanal
[0025] Add ground PCC (12.9 g, 60.0 mmol), 12.9 g of silica gel and 90.3 mL of anhydrous carbon dichloride into a 500 mL standard ground three-neck flask equipped with a reflux condenser. Under the protection of nitrogen, 1,7-heptanediol (6.58g, 49.85mmol) was added quickly and kept stirring, reacted at room temperature for 1h, then added 27.1mL of distilled water and 90.3mL of diethyl ether for extraction, stirred for 50min, and then used Silica gel suction filtration, transfer the filtrate to a separatory funnel, add 20% sodium hydroxide dropwise to make the filtrate neutral, extract three times with ether and combine the organic phases, wash three times with saturated sodium chloride, dry over anhydrous sodium sulfate for 12 hours, evaporate The solvent was removed, and the residue was separated with a silica gel column to obtain 4.53 g of 7-hydroxy-1-heptanal, with a yield of 68.6%.
[0026] (2) Synthesis of Z7,9-decadien-1-...
Embodiment 3
[0029] (1) Synthesis of 7-hydroxyl-1-heptanal
[0030] Add ground PCC (16.0g, 77.4mmol), 16.0g of silica gel and 136mL of anhydrous carbon dichloride into a 500mL standard ground three-neck flask equipped with a reflux condenser, and stir thoroughly for 30 min. , add 1,7-heptanediol (9.0g, 68.18mmol) quickly, and keep stirring, react at room temperature for 2h, then add 34mL of distilled water and then add 130mL of diethyl ether for extraction, and then stir for 40min, and then use silica gel to filter the filtrate Transfer to a separatory funnel, add 20% sodium hydroxide dropwise to make the filtrate neutral, extract three times with ether and combine the organic phases, wash three times with saturated sodium chloride, dry over anhydrous sodium sulfate for 12 hours, evaporate the solvent, and the residue is washed with silica gel Column separation yielded 6.45 g of 7-hydroxyl-1-heptanal, with a yield of 71.7%.
[0031] (2) Synthesis of Z7,9-decadien-1-ol
[0032] In a 250mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com