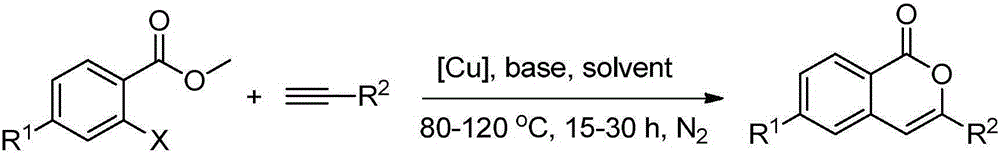
Method for preparing isocoumarin compounds with o-halo methyl benzoate and terminal alkyne
A technology of methyl benzoate and terminal alkyne, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions and low product selectivity, and achieve the effects of simple reaction operation, multiple types and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0024] Synthesis of 3-Phenylisocoumarin
[0025] Add 0.22mmol methyl o-bromobenzoate, 0.20mmol phenylacetylene, 0.02mmol cuprous iodide, 0.40mmol sodium hydroxide, and 1.0mL acetonitrile solvent into the reactor. Under nitrogen atmosphere, heat to 100°C, keep stirring for 24h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution for washing, extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 96%. 1 HNMR (400MHz, CDCl 3 ):δ8.31(d,J=8.1Hz,1H),7.88(d,J=6.7Hz,2H),7.72(t,J=7.6Hz,1H),7.51-7.40(m,5H),6.96 (s,1H).
Synthetic example 2
[0027] Synthesis of 3-(2-methylphenyl)isocoumarin
[0028] Add 0.20mmol methyl o-bromobenzoate, 0.20mmol 2-ethynyltoluene, 0.02mmol cuprous iodide, 0.40mmol sodium hydroxide, and 1.0mL acetonitrile solvent into the reactor. Under nitrogen atmosphere, heat to 100°C, keep stirring for 24h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution for washing, extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 85%. 1 HNMR (400MHz, CDCl 3 ): δ8.31(d, J=7.9Hz, 1H), 7.72(t, J=7.5Hz, 1H), 7.54-7.44(m, 3H), 7.33(t, J=7.4Hz, 1H), 7.30 -7.20(m,2H),6.60(s,1H),2.50(s,3H).
Synthetic example 3
[0030] Synthesis of 3-(4-tert-butylphenyl)isocoumarin
[0031] Add 0.24mmol methyl o-bromobenzoate, 0.20mmol p-tert-butylphenylacetylene, 0.02mmol cuprous iodide, 0.40mmol sodium hydroxide, and 1.0mL acetonitrile solvent into the reactor. Under nitrogen atmosphere, heat to 100°C, keep stirring for 24h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution for washing, extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 95%. 1 HNMR (400MHz, CDCl 3 ):δ8.30(d,J=7.9Hz,1H),7.82(d,J=8.4Hz,2H),7.70(m,1H),7.49-7.44(m,3H),6.92(s,1H) ,1.35(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com