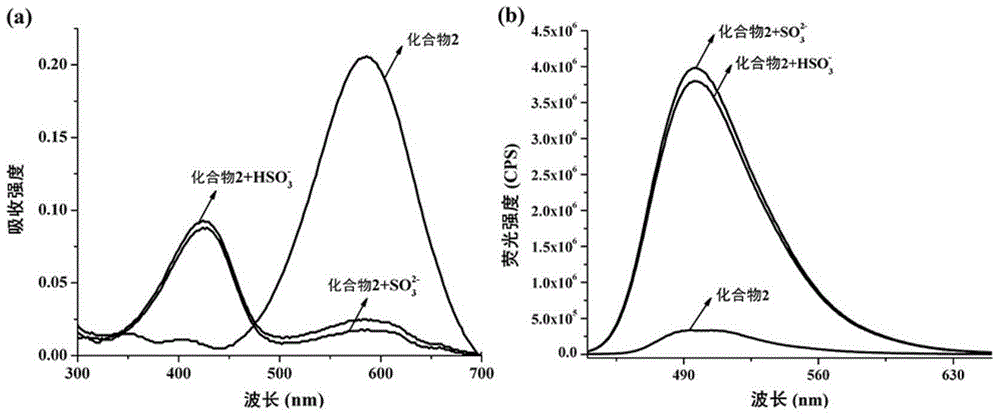
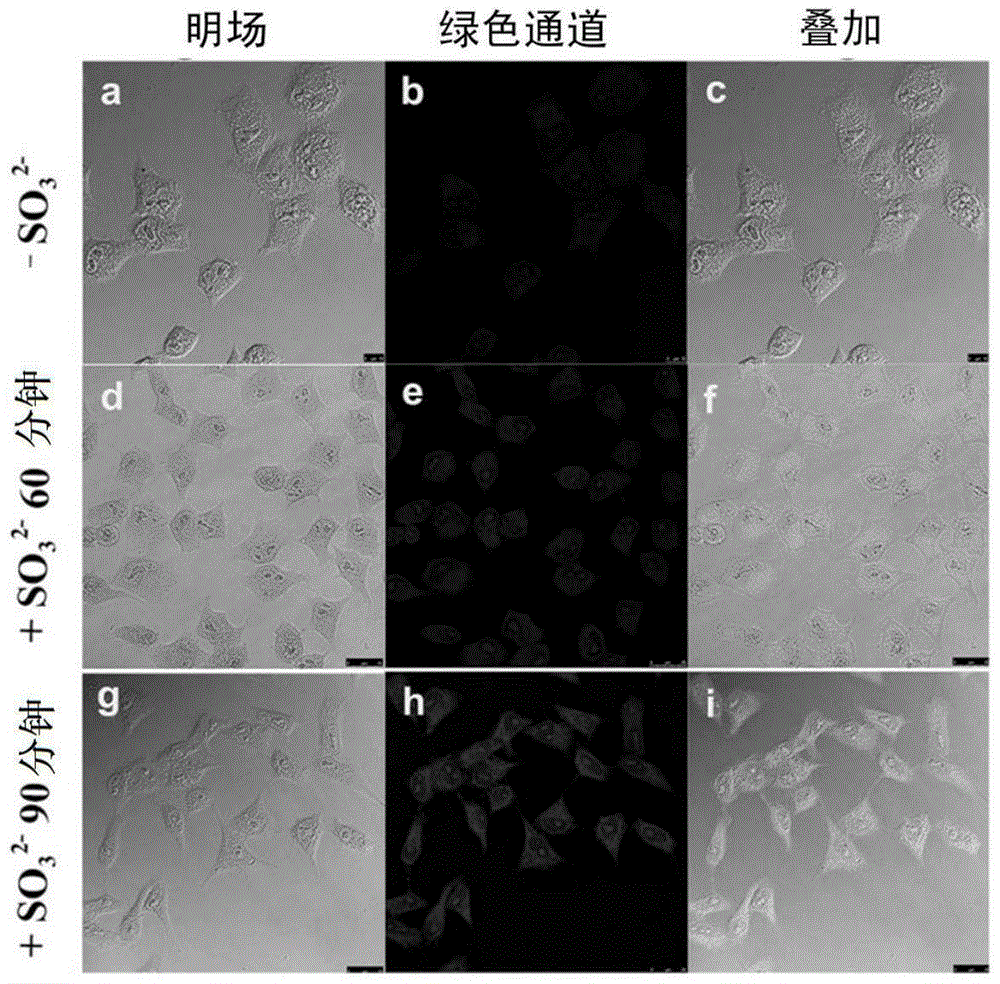
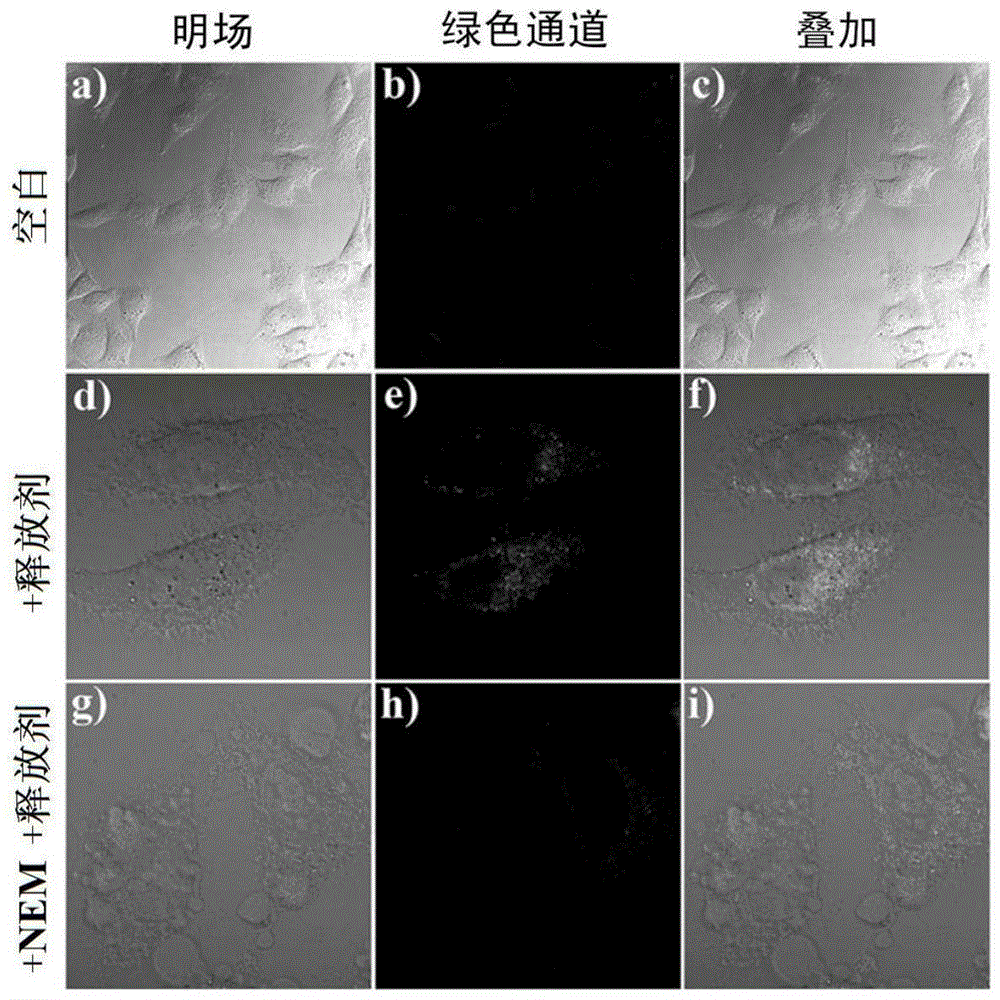
Substitution coumarin-pyridine derivative, preparing method thereof and application thereof
A technology of coumarin and derivatives, applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problems of small Stokes shift and low fluorescence quantum yield, and achieve large Stokes shift, The effect of simple and easy to obtain raw materials and excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the substituted coumarin-pyridine derivative comprises the following steps:
[0029] a. Dissolve 3-cyano-4-methylpyridine and methyl iodide in toluene, stir at room temperature for 3 to 6 hours and then reflux for 20 to 40 minutes to prepare intermediate 1; the amount of methyl iodide 1.1 to 1.5 times the equivalent of 3-cyano-4-methylpyridine;
[0030] b. Dissolving the substituted coumarin aldehyde and intermediate 1 in acetic anhydride, and reacting under reflux for 3-6 hours to prepare a substituted coumarin-pyridine derivative. The amount of the intermediate 1 is 2 to 2.5 times the equivalent of the substituted coumarin aldehyde.
[0031] In the embodiment of the present invention, HeLa cell line was purchased from ATCC (American Type Culture Collection), 10% fetal bovine serum was purchased from Hyclone Company, and DMEM (H) medium was purchased from Gibco Company of the United States. The nuclear dye NucBlue and the mitochondrial dye M...
Embodiment 13
[0032] Synthesis of Example 13-cyano-4-methyl-1-picoline (intermediate 1)
[0033]
[0034] 3-Cyano-4-picoline (5.9 g, 50 mmol) and iodomethane (10.8 g, 55 mmol) were dissolved in toluene, and the reaction was stirred at room temperature for 4 hours and then refluxed for 30 minutes. After cooling the reaction solution, it was filtered with suction, and the obtained solid was washed with ether and dried to obtain 9.65 g (37.1 mmol) of a light yellow solid with a yield of 74.2%.
[0035] 1 HNMR (400MHz, DMSO): δ9.63(s, 1H), 9.09(d, J=6.4Hz, 1H), 8.25(d, J=6.4Hz, 1H), 4.31(s, 3H), 2.78(s ,3H).
Embodiment 23
[0036] Synthesis of Example 23-(Iodide 1-methyl-3-cyano-pyridine vinyl)-7-cyclohexanediaminocoumarin (compound 2)
[0037]
[0038] The compound cyclohexanediaminocoumarin aldehyde (53mg, 0.2mmol) and intermediate 1 (104mg, 0.4mmol) were dissolved in 5mL of acetic anhydride, refluxed and stirred at 120°C for 4 hours, after the reaction was complete, cooled to room temperature for pumping Filter, wash with ice ethanol and dry to obtain 72.1 mg (0.14 mmol) of a dark green solid with a yield of 70.5%.
[0039] 1 HNMR (400MHz, DMSO): δ9.45(s, 1H), 8.87(d, J=6.8Hz, 1H), 8.50(d, J=7.0Hz, 1H), 8.15(t, J=7.6Hz, 2H ), 7.85(d,J=15.4Hz,1H), 7.22(s,1H), 4.18(s,3H), 3.40(s,4H), 2.75(d,J=12.4,6.2Hz,4H), 1.92 (s,4H).
[0040] 13 CNMR (101MHz, DMSO): δ159.86(s), 154.79(s), 152.13(s), 150.63(s), 149.27(s), 148.55(s), 146.24(s), 143.48(s), 127.65 (s), 120.93(s), 120.47(s), 117.56(s), 114.09(s), 111.89(s), 109.13(s), 107.89(s), 105.54(s), 50.33(s), 49.77(s), 47.33(s), 27.16(s), 20.96(s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com