A kind of substituted phenyl indoline carbazole derivative and its preparation method and application
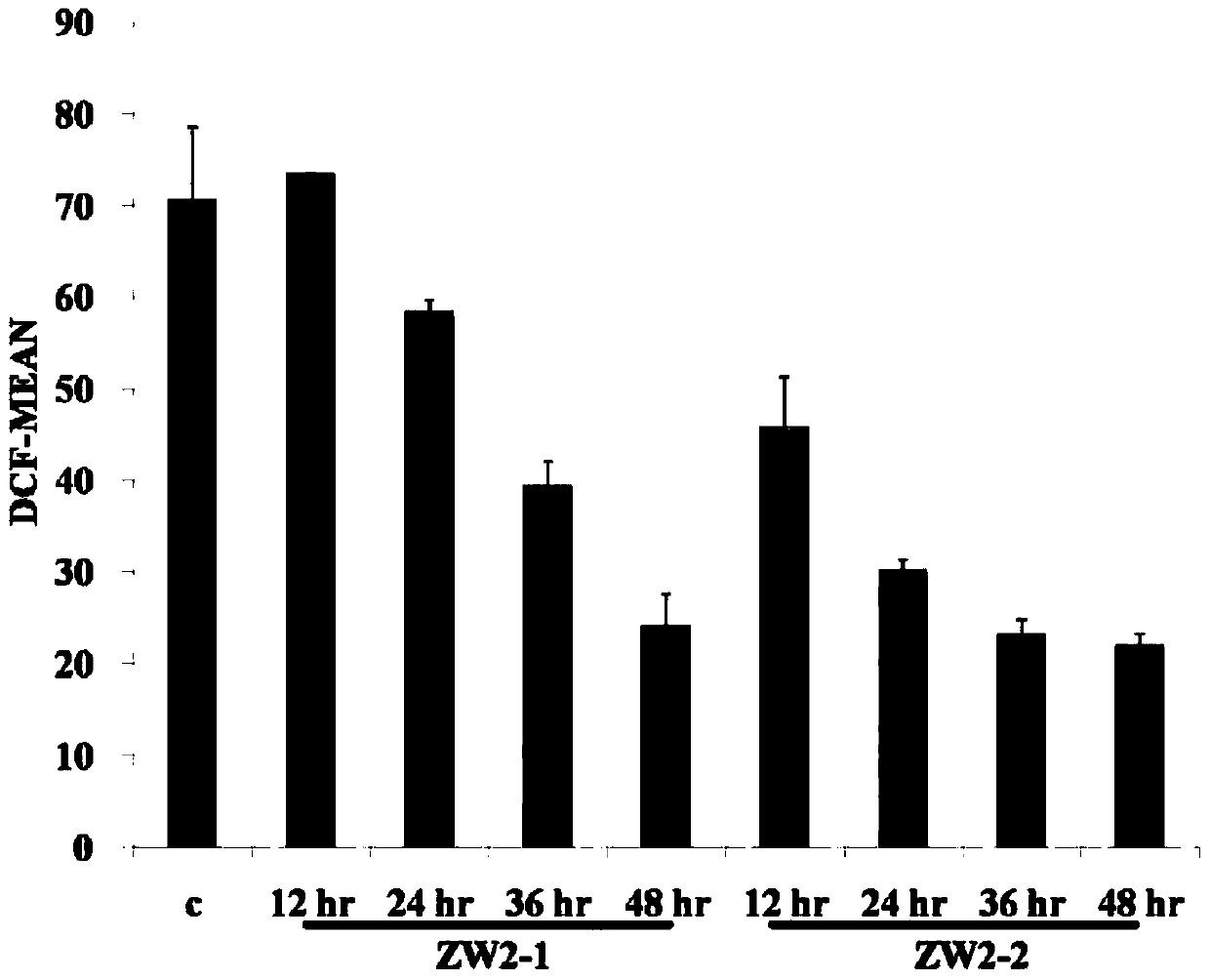
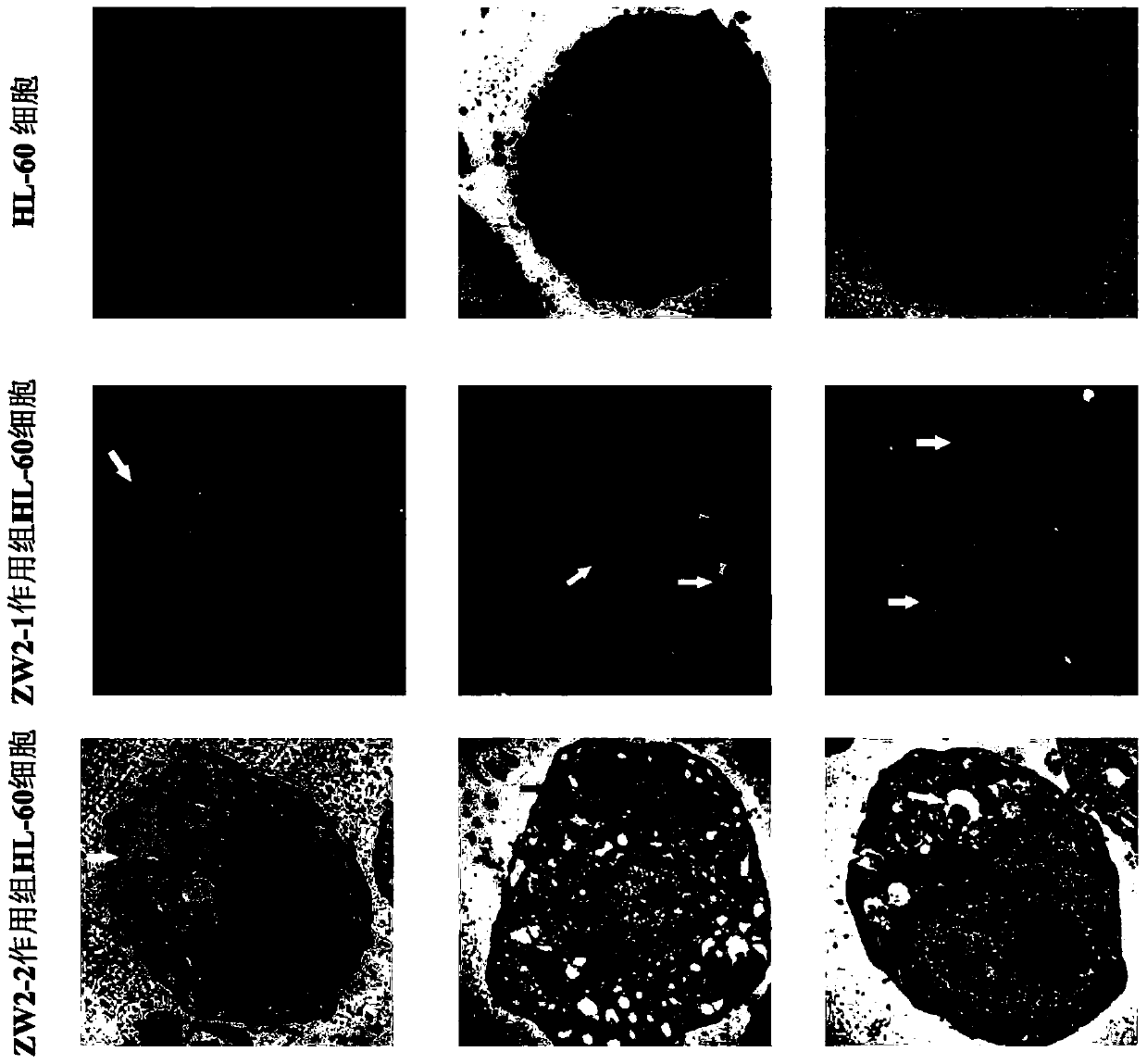
A technology of substituted phenylindolecarbazole and phenylindolecarbazole, which is applied in the field of substituted phenylindolecarbazole derivatives and their preparation, can solve the problem of insensitivity to chemotherapeutic drugs and resistance to It can reduce the level of ROS, induce apoptosis and autophagy, and achieve the effect of simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
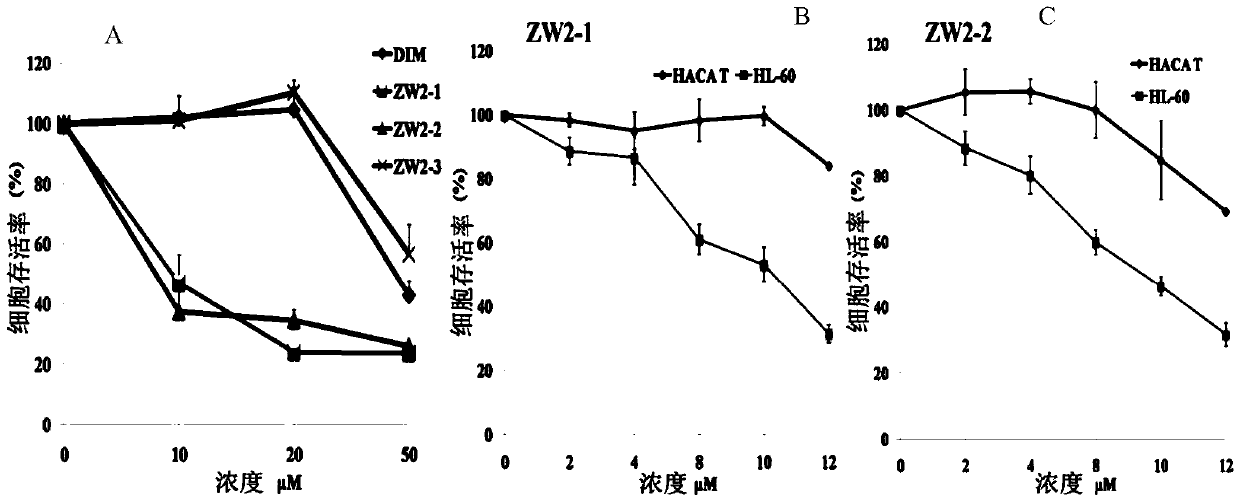
[0041] Embodiment 1: Preparation of indoline carbazole phenol (Ar is p-hydroxyphenyl, numbering is ZW2-1)
[0042] Add 492 mg (2 mmol) of bis-indolylmethane and 146 mg (1.2 mmol) of p-hydroxybenzaldehyde into a 50 mL round bottom flask, add 30 mL of absolute ethanol and stir to dissolve. Add 0.2 mL of concentrated sulfuric acid dropwise under ice-cooling, and stir thoroughly for 5 minutes. After reacting at room temperature for 24 hours, it was detected by TLC that the starting point of bisindolylmethane disappeared, the reaction was stopped, and the reaction solution was concentrated to dryness under reduced pressure. The residue was dissolved in 30mL ethyl acetate, transferred to a 100mL separatory funnel, washed successively with saturated aqueous sodium bicarbonate (10mL×3) and saturated aqueous sodium chloride (10mL×3), and the ethyl acetate layer was washed with anhydrous Dry over sodium sulfate for 2 hours, filter, and concentrate the filtrate to dryness under reduced ...
Embodiment 2
[0044] Embodiment 2: Preparation of indoline carbazole phenol (Ar is 3-carboxy-4-hydroxyl phenyl, numbering is ZW2-2)
[0045] Add 492mg (2mmol) of bisindolylmethane and 218mg (1.2mmol) of syringaldehyde into a 50mL round bottom flask, add 30mL of absolute ethanol and stir to dissolve. Add 0.2 mL of concentrated sulfuric acid dropwise under ice-cooling, and stir thoroughly for 5 minutes. After reacting at room temperature for 24 hours, it was detected by TLC that the starting point of bisindolylmethane disappeared, the reaction was stopped, and the reaction solution was concentrated to dryness under reduced pressure. The residue was dissolved in 30mL ethyl acetate, transferred to a 100mL separatory funnel, washed successively with saturated aqueous sodium bicarbonate (10mL×3) and saturated aqueous sodium chloride (10mL×3), and the ethyl acetate layer was washed with anhydrous Dry over sodium sulfate for 2 hours, filter, and concentrate the filtrate to dryness under reduced pr...
Embodiment 3
[0047] Example 3: Preparation of indoline carbazole phenol (Ar is 3,5-dimethoxy-4-hydroxyphenyl, numbered ZW2-3)
[0048] Add 492mg (2mmol) of bisindolylmethane and 180mg (1.1mmol) of salicylaldehyde into a 50mL round bottom flask, add 30mL of absolute ethanol and stir to dissolve. Add 0.2 mL of concentrated sulfuric acid dropwise under ice-cooling, and stir thoroughly for 5 minutes. After reacting at room temperature for 24 hours, it was detected by TLC that the starting point of bisindolylmethane disappeared, the reaction was stopped, and the reaction solution was concentrated to dryness under reduced pressure. The residue was dissolved in 30mL ethyl acetate, transferred to a 100mL separatory funnel, washed successively with saturated aqueous sodium bicarbonate (10mL×3) and saturated aqueous sodium chloride (10mL×3), and the ethyl acetate layer was washed with anhydrous Dry over sodium sulfate for 2 hours, filter, and concentrate the filtrate to dryness under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com