Erdosteine composition dry suspension for treating respiratory system disease
A technology for respiratory diseases and dry suspensions, applied in the field of medicine, can solve the problems of drug absorption speed or degree, poor water solubility of erdosteine, slow drug dissolution speed, etc., and achieve high stability and good stability , good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Erdosteine Crystals
[0024] Dissolve erdosteine in a mixed solvent of methanol and dimethyl sulfoxide whose volume is 6 times the weight of erdosteine at 40°C. The volume ratio of methanol to dimethyl sulfoxide is 5:3.5; Add a mixed solvent of ethanol and ether whose volume is 10 times the weight of erdosteine at a speed of 2:1, stir while adding, control the temperature at 40°C, and grow the crystal for 3 hours; then Add ethanol and dichloromethane whose total volume is 8 times the weight of erdosteine at a speed of 20ml / min. After growing the crystal for 1 hour, cool down to -5°C at a speed of 10°C / hour, and then keep stirring at 110 rpm Stirring per minute for crystallization and crystal growth for 4 hours; filtering, washing, and drying under reduced pressure to obtain erdosteine crystal compound.
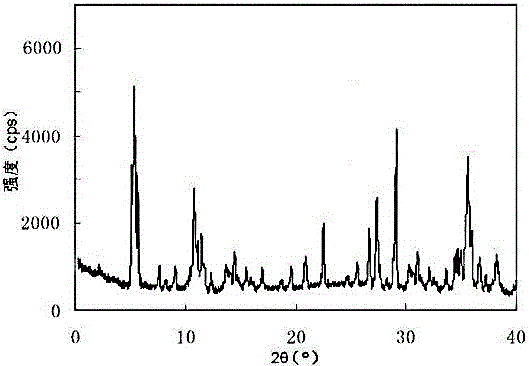
[0025] The X-ray powder diffraction pattern obtained by measuring the obtained erdosteine crystal using Cu-Kα ray is as follo...
Embodiment 2
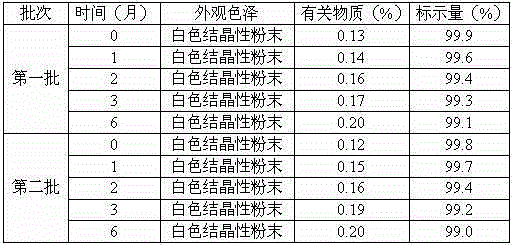
[0026] Example 2: Preparation of Erdosteine Dry Suspension
[0027] Prescription: 1.5 parts by weight of the erdosteine crystal compound prepared in Example 1, 2.3 parts by weight of sorbitol, 0.08 parts by weight of potassium metaphosphate, 0.15 parts by weight of gum arabic, 0.015 parts by weight of glycyrrhizin .
[0028] Preparation:
[0029] (1) Processing of raw and auxiliary materials: sieve erdosteine and potassium metaphosphate to 80 mesh;
[0030] (2) Weighing: Weighing according to the prescription;
[0031] (3) Mixing: Put erdosteine, potassium metaphosphate, sorbitol, gum arabic, and glycyrrhizin into the three-dimensional motion mixer, set the pre-mixing speed to 15 rpm, and the mixing time to 30 minutes;
[0032] (4) Packaging: Calculate the theoretical loading range according to the content of the materials obtained from the total blending, and then carry out sub-packaging.
Embodiment 3
[0033] Example 3: Preparation of Erdosteine Dry Suspension
[0034] Prescription: 1.5 parts by weight of the erdosteine crystal compound prepared in Example 1, 2.4 parts by weight of sorbitol, 0.1 parts by weight of potassium metaphosphate, 0.2 parts by weight of gum arabic, 0.02 parts by weight of glycyrrhizin .
[0035] Preparation:
[0036] (1) Processing of raw and auxiliary materials: sieve erdosteine and potassium metaphosphate to 80 mesh;
[0037] (2) Weighing: Weighing according to the prescription;
[0038] (3) Mixing: Put erdosteine, potassium metaphosphate, sorbitol, gum arabic, and glycyrrhizin into the three-dimensional motion mixer, set the pre-mixing speed to 15 rpm, and the mixing time to 30 minutes;
[0039] (4) Packaging: Calculate the theoretical loading range according to the content of the materials obtained from the total blending, and then carry out sub-packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com