G-C3N4 supported cobalt oxide catalyst and preparation method thereof
A g-c3n4, cobalt oxide technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, separation methods, etc., to achieve excellent catalytic low-temperature oxidation activity of carbon monoxide, high stability, and excellent electron transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
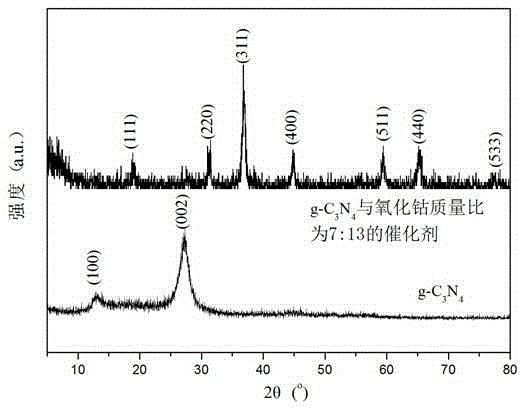
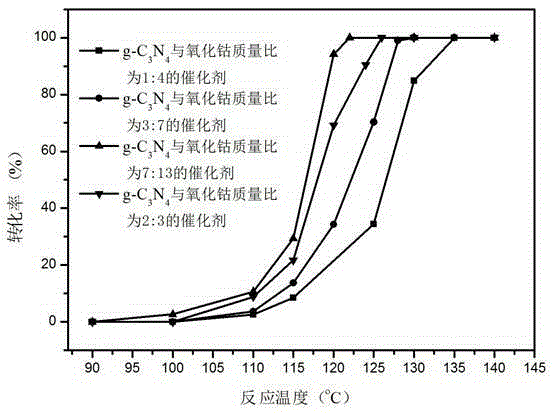
Image
Examples
preparation example Construction
[0022] a g-C 3 N 4 The preparation method of supported cobalt oxide catalyst comprises the following steps:
[0023] Step 1. At room temperature, take cobalt acetate and distilled water according to the mass ratio of 1:15-16; add the taken cobalt acetate into the distilled water, and magnetically stir it to completely dissolve it to obtain an aqueous solution of cobalt acetate, which is set aside;
[0024] Step 2, follow g-C 3 N 4 The mass ratio of the powder to the cobalt oxide taken in the above step 1 is the ratio of 1:4-2:3, taking g-C 3 N 4 powder; take the g-C 3 N 4 Add the powder to distilled water twice the volume of the distilled water taken in the above step 1, and place it in an ultrasonic reactor with a power of 168W for 60-120 minutes to obtain g-C 3 N 4 aqueous solution, spare;
[0025] Step 3, the prepared cobalt acetate aqueous solution and g-C 3 N 4 The aqueous solution is mixed, stirred magnetically for 20-25 minutes to obtain a mixed solution A; t...
Embodiment 1
[0033] At room temperature, take 6.5 grams of cobalt acetate and dissolve it in 100 ml of distilled water, and stir it magnetically to dissolve it completely to obtain cobalt acetate aqueous solution I, which is set aside; 3 N 4 Take the corresponding amount of g-C in the ratio of 1:4 to the mass ratio of cobalt oxide 3 N 4 The powder was added to 200ml of distilled water and ultrasonicated for 60 minutes to obtain g-C 3 N 4 Aqueous solution II, spare; the g-C mentioned therein 3 N 4 Using melamine as the precursor, the temperature is raised to 520°C at a rate of 10°C / min, and the g-C is prepared in a block for 4 hours. 3 N 4 , after grinding, sieving, and set aside. Mix solution I and solution II, stir magnetically for 20 minutes, add 12 ml of ammonia water with a mass percentage of 28% dropwise, and stir magnetically for 120 minutes; put the mixed solution into a high-pressure reactor lined with polytetrafluoroethylene , hydrothermal reaction at 150°C for 10 hours, a...
Embodiment 2
[0035] At room temperature, take 6.5 grams of cobalt acetate and dissolve it in 100 ml of distilled water, and stir it magnetically to dissolve it completely to obtain cobalt acetate aqueous solution I, which is set aside; 3 N 4 Take the corresponding amount of g-C in the ratio of 3:7 to the mass ratio of cobalt oxide 3 N 4 The powder was added to 200ml of distilled water and ultrasonicated for 80 minutes to obtain g-C 3 N 4 Aqueous solution II, spare; the g-C mentioned therein 3 N 4 Using melamine as the precursor, the temperature is raised to 520°C at a rate of 10°C / min, and the g-C is prepared in a block for 4 hours. 3 N 4 , after grinding, sieving, and set aside. Mix solution I and solution II, stir magnetically for 20 minutes, add 12 ml of ammonia water with a mass percentage of 28% dropwise, and stir magnetically for 120 minutes; put the mixed solution into a high-pressure reactor lined with polytetrafluoroethylene , hydrothermal reaction at 150°C for 10 hours, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com