Lithium iron phosphate cathode material with carbon coating in situ and preparation method thereof
A carbon-coated lithium iron phosphate and cathode material technology, applied in the fields of nanomaterials and electrochemistry, can solve the problems of low electrode material capacity, poor high rate performance, short cycle life, etc., and achieve low charge mass transfer resistance and discharge capacity. High, growth-inhibiting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
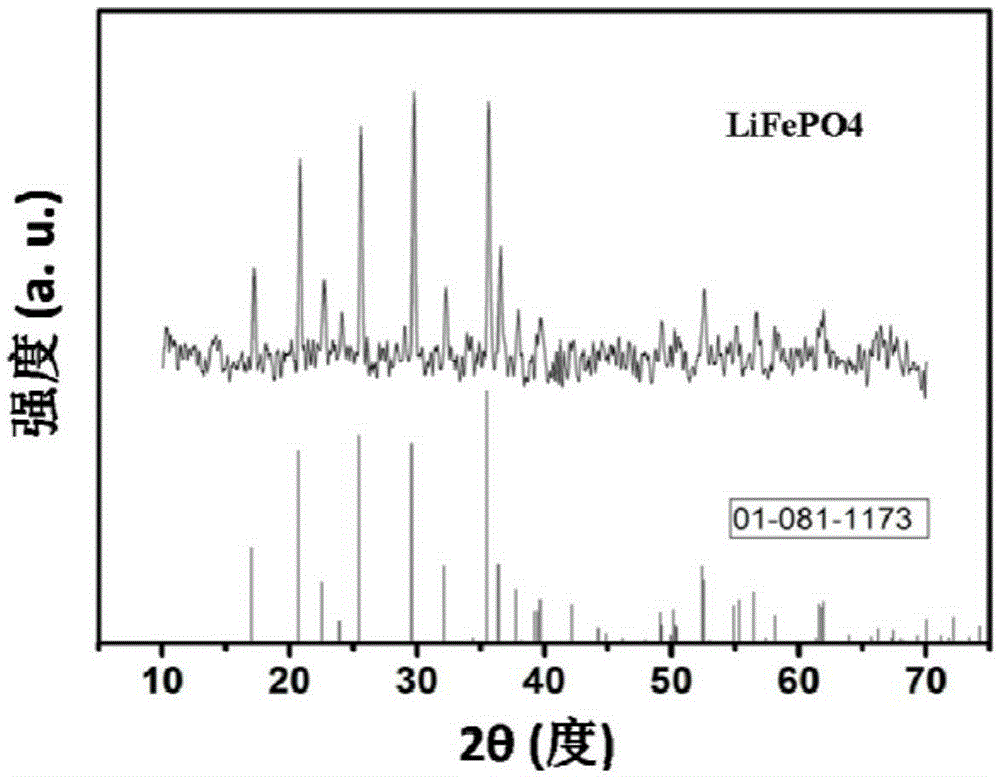
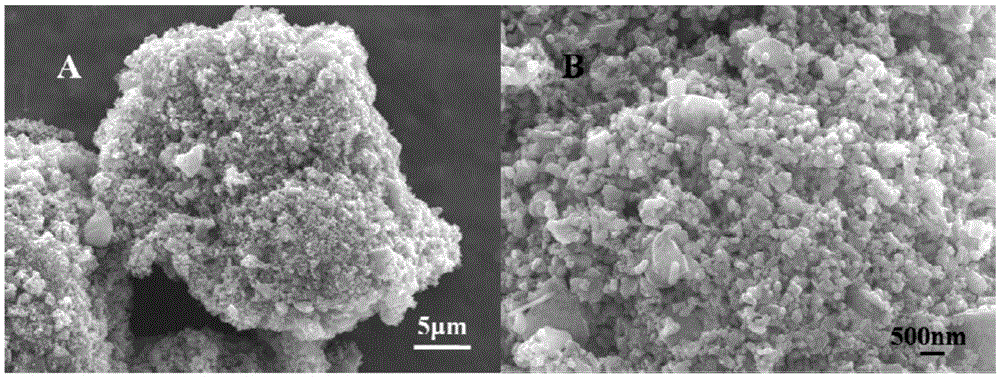
[0030] Example 1, see Figure 1 to Figure 5 Shown:
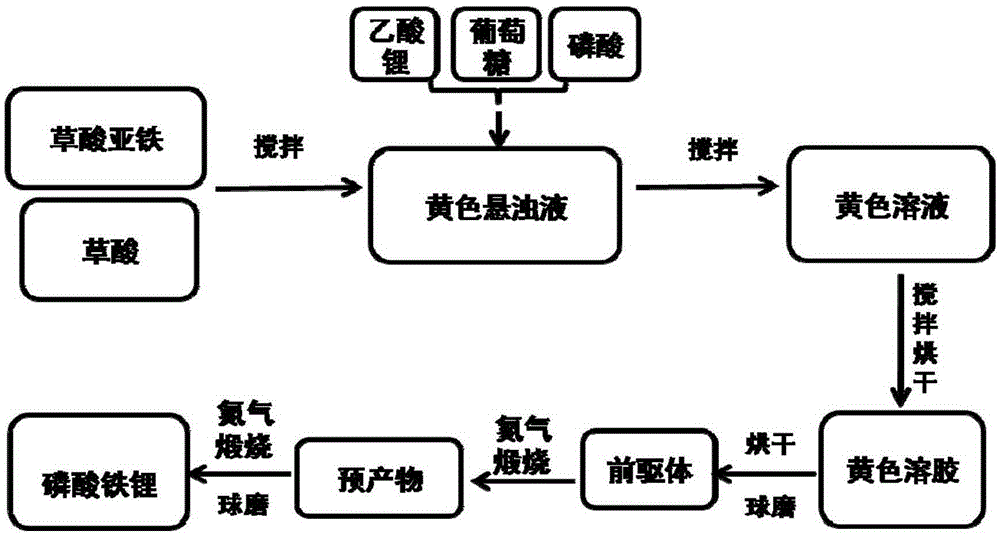
[0031] The invention provides an in-situ carbon-coated lithium iron phosphate positive electrode material and a preparation method thereof, comprising the following steps (such as figure 1 shown):
[0032] 1) 1.26g ferrous oxalate dihydrate (FeC 2 o 4 2H 2 O), 2.56g oxalic acid (C 2 h 2 o 4 2H 2 O) join in 20mL distilled water, form yellow suspension solution;
[0033] 2) 0.75g lithium acetate (CH 3 COOLi·2H 2 O) was dissolved in 10 mL of distilled water to form a clear solution; 0.3 g of glucose (C 6 h 12 o 6 ·H 2 O) Dissolve in 10mL distilled water to form a clear solution; respectively add lithium acetate solution, 480 μL phosphoric acid, and glucose solution into the above yellow suspension (the molar ratio of lithium source to phosphorus source is 1.05:1, the actual amount of lithium source is the required 1.05 times the reaction volume);
[0034] 3) After stirring the above yellow suspension at 80°C for...
Embodiment 2
[0042] 1) 1.26g ferrous oxalate dihydrate (FeC 2 o 4 2H 2 O), 1.28g oxalic acid (C 2 h 2 o 4 2H 2 O) join in 20mL distilled water, form yellow suspension solution;
[0043] 2) 0.3084g lithium hydroxide monohydrate (LiOH·H 2 O) was dissolved in 10 mL of distilled water to form a clear solution; 0.3 g of sucrose (C 12 h 22 o 11 ) was dissolved in 10mL distilled water to form a clear solution; lithium hydroxide solution, 480μL phosphoric acid, and sucrose solution were added to the above yellow suspension (the molar ratio of lithium source to phosphorus source was 1.05:1, and the actual amount of lithium source was the required 1.05 times the reaction volume);
[0044] 3) After stirring the above yellow suspension at 90°C for 36h, the yellow suspension turned into a yellow solution; the yellow solution was stirred and dried to obtain a yellow sol; the solid obtained after drying the yellow sol at 120°C was placed in Ball milling for 6 hours to obtain reddish-brown prec...
Embodiment 3
[0048] 1) 1.26g ferrous oxalate dihydrate (FeC 2 o 4 2H 2 O), 5.12g oxalic acid (C 2 h 2 o 4 2H 2 O) join in 20mL distilled water, form yellow suspension solution;
[0049] 2) 0.75g lithium acetate (CH 3 COOLi·2H 2 O) was dissolved in 10 mL of distilled water to form a clear solution; 0.8051 g of ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) was dissolved in 10mL distilled water to form a clear solution; 0.3g glucose (C 6 h 12 o 6 ·H 2 O) Dissolve in 10mL distilled water to form a clear solution; respectively add lithium acetate solution, ammonium dihydrogen phosphate solution, and glucose solution into the above-mentioned yellow suspension (the molar ratio of lithium source to phosphorus source is 1.05:1, the actual amount of lithium source 1.05 times the required reaction volume);
[0050] 3) After stirring the above yellow suspension at 95°C for 12 hours, the yellow suspension turned into a yellow solution; the yellow solution was stirred and dried to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com