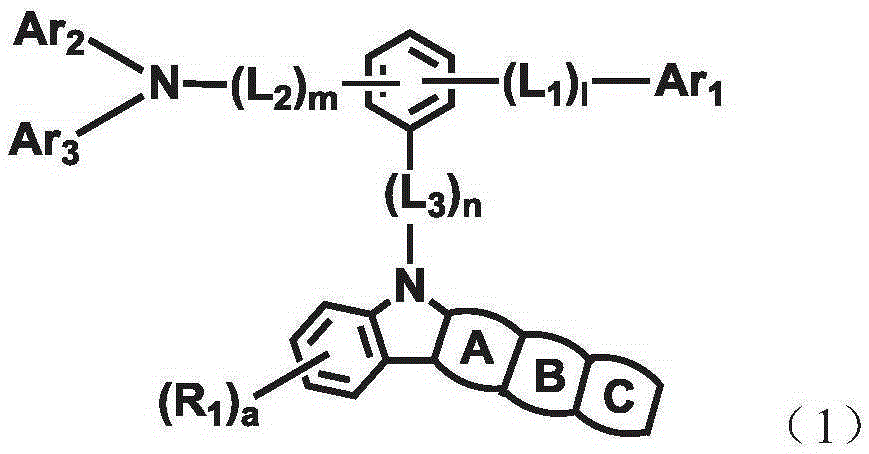
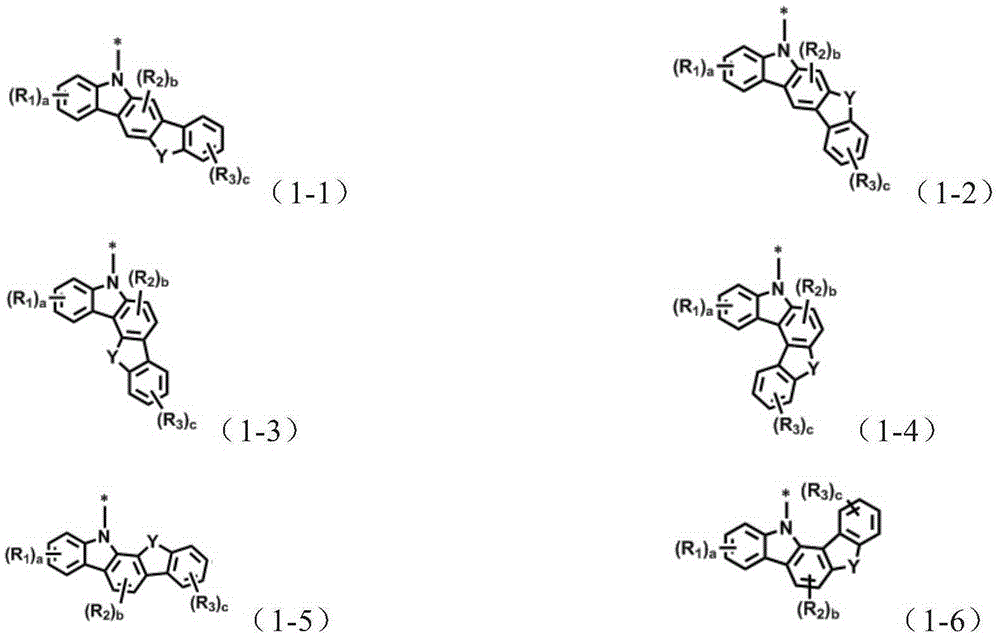
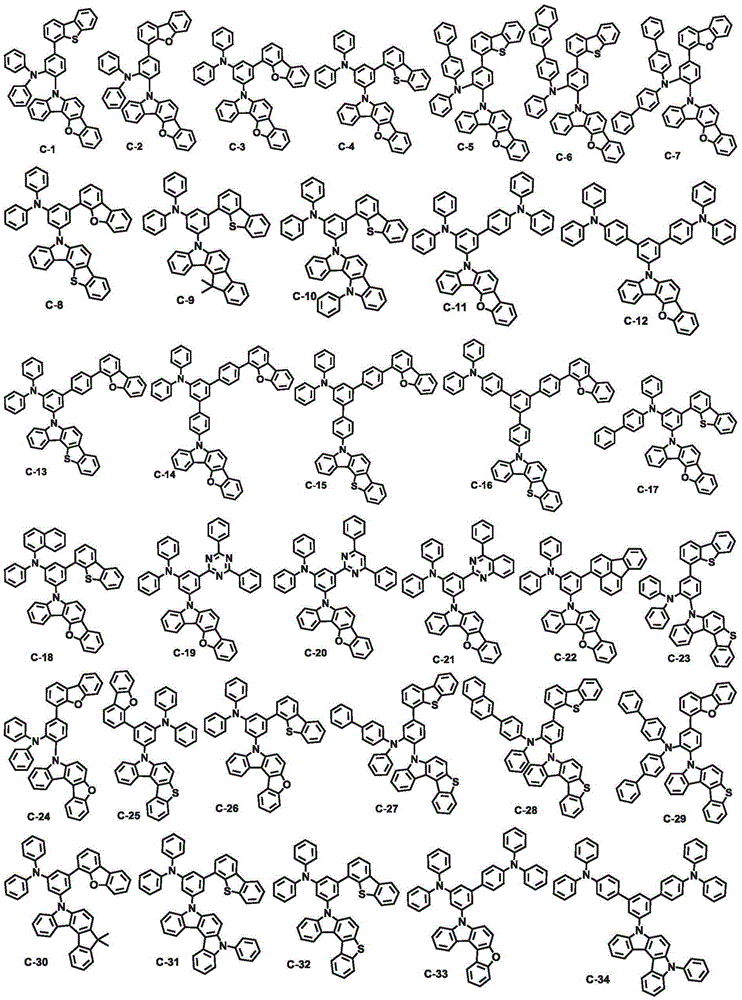
Organic electroluminescent compound and organic electroluminescent device comprising the same
An electroluminescence device and electroluminescence technology, which are applied in the fields of organic chemistry, light-emitting materials, chemical instruments and methods, etc., can solve the problem of high hole mobility, low glass transition temperature, and reduced hole-electron charge balance quantum yield. etc., to achieve the effect of high current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0105] Example 1: Preparation of Compound C-1
[0106]
[0107] Preparation of compound 1-1
[0108] In 5H-benzofuro[3,2-c]carbazole (30g, 117mmol), 4-bromo-1-fluoro-2-nitrobenzene (26g, 117mmol), cesium carbonate (46g, 140mmol) and After 300 mL of dimethyl sulfoxide was introduced into the reaction vessel, the mixture was stirred at room temperature overnight. The mixture was diluted with ethyl acetate, washed several times with water, and dried over anhydrous magnesium sulfate. The mixture was distilled under reduced pressure, and purified by column chromatography to obtain Compound 1-1 (45 g, 85%).
[0109] Preparation of Compound 1-2
[0110] After compound 1-1 (30 g, 66 mmol), tin(II) chloride hydrate (44.4 g, 200 mmol), and 700 mL of ethyl acetate were introduced into the reaction vessel, the mixture was refluxed for 2 hours. After cooling to room temperature, the mixture was diluted with ethyl acetate, washed with water several times, and dried over anhydrou...
example 2
[0116] Example 2: Preparation of Compound C-4
[0117]
[0118] Preparation of compound 2-1
[0119] In 5H-benzofuro[3,2-c]carbazole (30g, 117mmol), 1-bromo-3,5-dichlorobenzene (40g, 176mmol), copper(I) iodide (11g, 59mmol ), ethylenediamine (15 mL, 234 mmol), potassium phosphate (50 g, 234 mmol), and 600 mL of toluene were introduced into the reaction vessel, and the mixture was refluxed overnight. The mixture was diluted with ethyl acetate, washed several times with water, and dried over anhydrous magnesium sulfate. The mixture was distilled under reduced pressure, and purified by column chromatography to obtain compound 2-1 (28 g, 60%).
[0120] Preparation of compound 2-2
[0121] In compound 2-1 (14.3g, 35.5mmol), diphenylamine (6g, 35.5mmol), palladium (II) acetate (0.16g, 0.71mmol), S-Phos (2-bicyclic phosphino-2', After 6'-dimethoxyphenyl) (0.58 g, 1.42 mmol), sodium tert-butoxide (4 g, 43 mmol) and 350 mL of o-xylene were introduced into the reaction vess...
example 3
[0125] Example 3: Preparation of Compound C-12
[0126]
[0127] Compound 2-1 (7.5g, 18.5mmol), 4-(diphenylamino)phenylboronic acid (12.3g, 42.5mmol), palladium (II) acetate (0.35g, 1.5mmol), S-Phos ( 2-bicyclic phosphino-2',6'-dimethoxyphenyl) (0.9 g, 2.2 mmol), potassium phosphate (12 g, 55.5 mmol), 100 mL of toluene, 25 mL of 1,4-dioxane and 25 mL of distilled water After introduction into the reaction vessel, the mixture was refluxed overnight. After completing the reaction, the mixture was washed with distilled water, extracted with ethyl acetate, and dried over magnesium sulfate. After removing the solvent by a rotary evaporator, the mixture was purified by column chromatography to obtain compound C-12 (9.4 g, 62%).
[0128] Physical properties: Melting point 178°C, UV354nm (in toluene), PL396nm (in toluene), molecular weight 821.59[M+1]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com