Synthesis method of 3-methyl-2-en-4-yn pentanol through acid ionic liquid catalysis
A technology of acidic ionic liquid and synthesis method, applied in the field of green chemistry, can solve the problems of undisclosed specific methods of catalyst recovery and regeneration, unreachable yield, environmental pollution, etc., and achieve less by-products, simple post-treatment, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
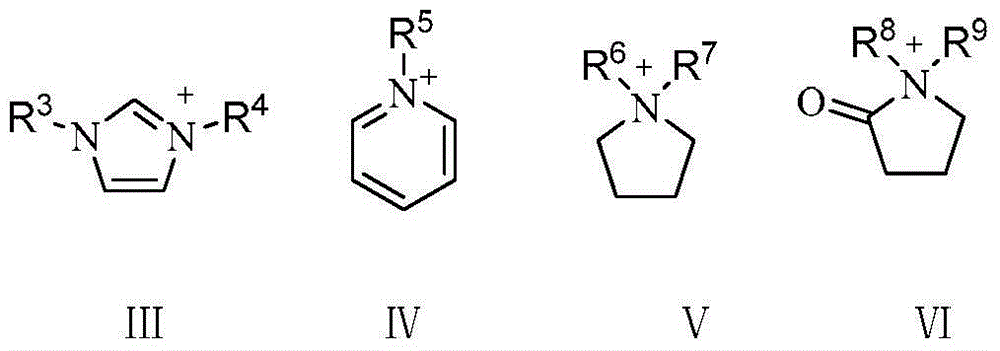
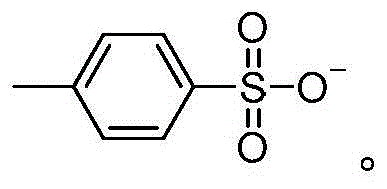
[0049]The synthesis steps of ionic liquid IL1-IL9 are as follows: N-methylimidazole, pyridine, N-methylpyrrole are equal to excess chlorobutane and refluxed in toluene for 48 hours, and the obtained chloride salts are respectively mixed with CH 3 COOK, KHSO 4 , Potassium p-toluenesulfonate, Potassium tetrafluoroborate, Potassium hexafluorophosphate, etc. for ion exchange reaction, the obtained product is filtered and desolubilized, CH 2 Cl 2 The structural formulas of IL1-IL9 and IL13-IL15 obtained by extraction and vacuum drying are as follows:
[0050]
Embodiment 13-15
[0052] The synthesis steps of ionic liquid IL10-IL12 are as follows: N-methyl-2-pyrrolidone, acetic acid, concentrated sulfuric acid, and p-toluenesulfonic acid were heated and reacted for 2 hours at a molar ratio of 1:1, and the product was washed three times with ethyl acetate after cooling , through vacuum drying, the product N-methylpyrrolidone acetate IL10, N-methylpyrrolidone bisulfate IL11, N-methylpyrrolidone p-toluenesulfonate IL12, the structural formula is as follows:
[0053]
Embodiment 16-33
[0055] Embodiment 16-33 has investigated under the action of different catalysts, 3-methyl-1-ene-4-yne-3-pentanol catalytic transposition generates 3-methyl-2-ene-4-ynylpentanol, the steps as follows:
[0056] Add 100g of 98% 3-methyl-1-en-4-yne-3-pentanol, 100ml of water, 100ml and 50g of protonic acid catalyst or ionic liquid in a 500ml flask, and keep the temperature of the material at about 50-60°C. Stir the reaction for 4~6h. GC tracks the progress of the reaction. After the reaction is complete, the reaction system is evacuated and rotary evaporated. The evaporated liquid is separated from the water layer, and the oil layer is washed with saturated Na 2 CO 3 Wash with aqueous solution for 1 to 2 times to obtain the crude product, and then obtain high content of E-3-methyl-2-ene-4-ynylpentanol and Z-3-methyl-2-ene-4-ynylpentanol . The ionic liquid phase is washed with an organic solvent and recycled. The results of each group of reactions are listed in Table 1. In or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com