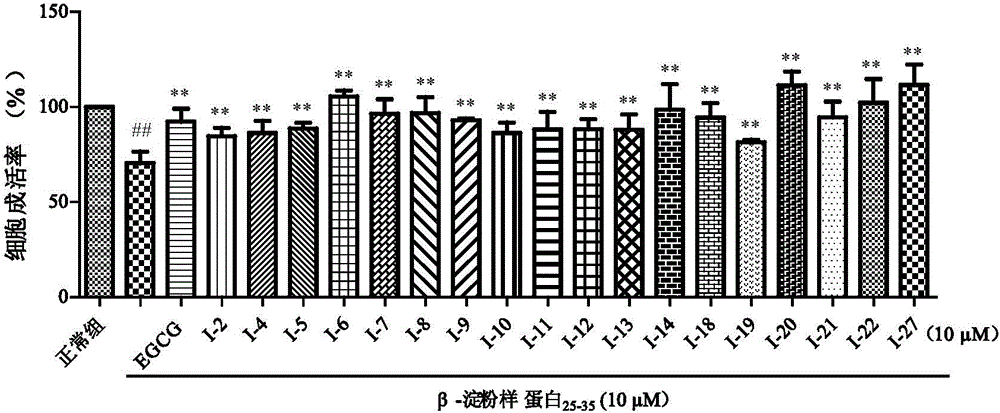
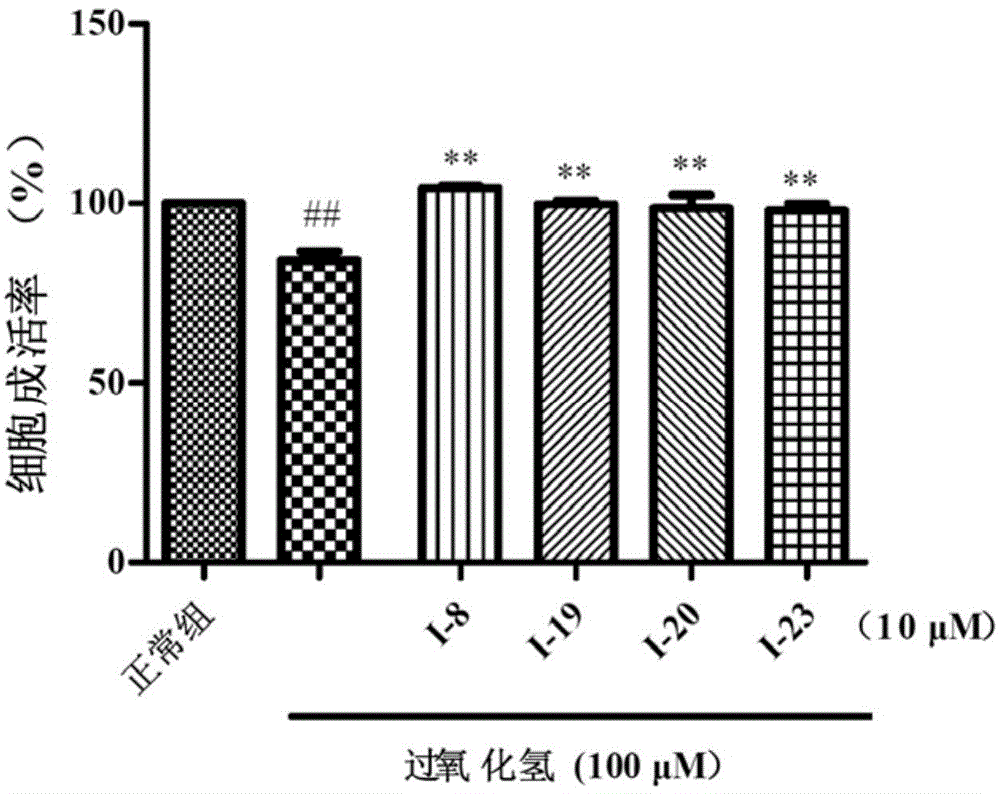
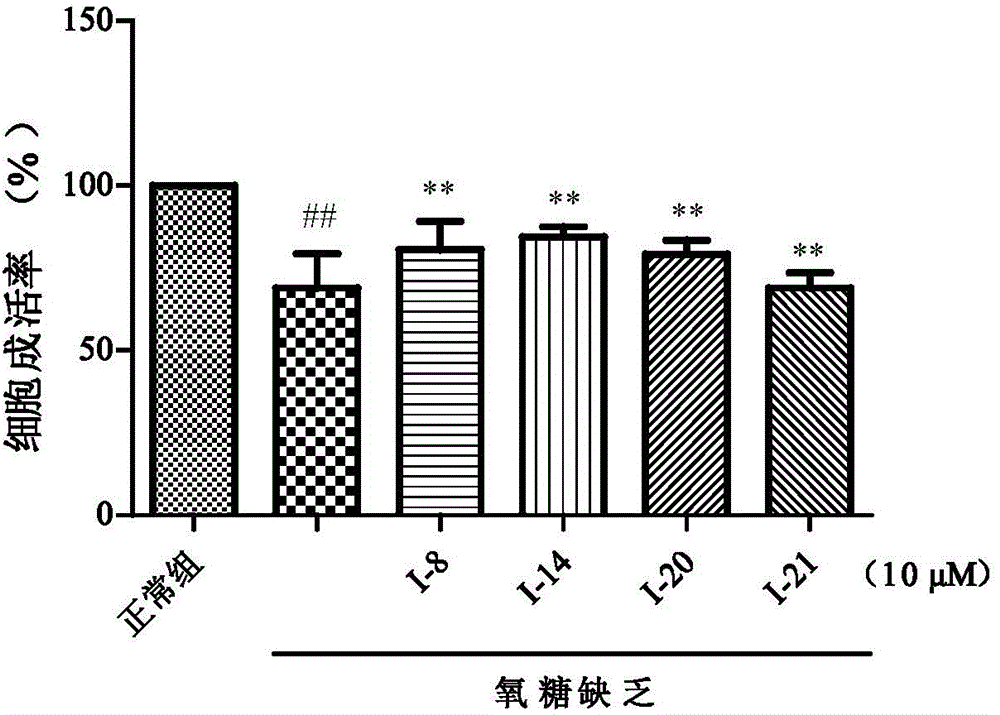
Indole alkaloids possessing 1,2,4-oxadiazole fragment, and preparation method and application thereof
A technology of alkaloids and indoles, applied in the field of medicine, can solve the problems of accelerating the onset of AD and achieve the effect of novel structure, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The preparation method of formula I compound of the present invention comprises the following steps:
[0090] (a) the compound of formula 1 is condensed with the compound of formula 2 to obtain the compound of formula 3;
[0091]
[0092] (b) the compound of formula 3 and the compound of formula 4 obtain the compound of formula I through coupling reaction,
[0093]
[0094] Among them, R in each formula 1 is bromine or iodine;
[0095] The definition of A, R and n is as described in claim 1;
[0096] R 3 It is a boric acid group or a borate ester group.
[0097] In another preferred example, the R 3 for -B(OH) 2 or
[0098] The condensing agent used in the step (a) is N,N'-dicyclohexylcarbodiimide (DCC), N,N'-diisopropylcarbodiimide (DIC), 1-ethyl- (3-Dimethylaminopropyl)carbodiimide hydrochloride (EDCI), diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), 1-hydroxy-7 -Azobenzotriazole (HOAT), 1-hydroxybenzotriazole (HOBT), O-(7-azoben...
Embodiment 1
[0127] The preparation of embodiment 1 formula 3 compound
[0128]
[0129] Dissolve 0.50g of the compound of formula 1 in 50mL of methanol, then add 1.20g of 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate and 0.8mLN , N-Diisopropylethylamine. After the mixture was stirred at room temperature for 0.5 hour, 0.59 g of the compound of formula 2 was added, and stirring was continued at room temperature for 1 hour. Afterwards, the reaction solution was concentrated, and the organic phase was extracted with ethyl acetate / water. The organic phase was washed successively with water and saturated brine, and concentrated. The residue was dissolved in 50 mL of ethanol and heated to reflux for 6 hours. After concentration, the residue was purified by column chromatography to obtain compound Formula 3 as a white solid with a yield of 75%.
[0130] 1 HNMR (300MHz, acetone-d6): δ7.62(d, J=9.0Hz, 1H), 7.43(m, 2H), 7.17(d, J=6.0Hz, 1H), 7.14(dd, J=8.0, 8.0Hz, ...
Embodiment 2
[0131] The preparation of embodiment 2 formula I compound
[0132]
[0133] Under the protection of Ar, 20 mg of the compound of formula 3 was dissolved in 3 mL of 1,4-dioxane, and then 4 mg of tetrakis(triphenylphosphine) palladium, 0.5 mL of water, 20 mg of potassium carbonate and the compound of formula 4 were added. The above mixture was heated at 90° C. for 12 hours, then concentrated, and the residue was purified by column chromatography to obtain the compound of formula I. The compound numbers, specific structural formulas and raw materials used are shown in Table 1 below.
[0134] Table 1 Compound number, specific structural formula and raw materials used
[0135]
[0136]
[0137]
[0138]
[0139] The yield of compound I-1 was 87%. 1 HNMR (300MHz, acetone-d6): δ7.84(d, J=90Hz, 2H), 7.65(d, J=6.0Hz, 1H), 7.50-7.35(m, 5H), 7.25(d, J=6.0 Hz,1H),7.14(dd,J=6.0,6.0Hz,1H),7.08(d,J=6.0Hz,1H),7.05(m,dd,J=6.0,6.0Hz,1H),4.53(s ,2H).ESI-MS: 342.2[M+H + ],364....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com