Type of isoquinoline as well as preparation method and application thereof
A technology of isoquinoline and dihydroxyisoquinoline, which is applied in the fields of resisting vector-borne diseases, antineoplastic drugs, organic chemistry, etc., can solve problems such as unclear active components of centipedes and application restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
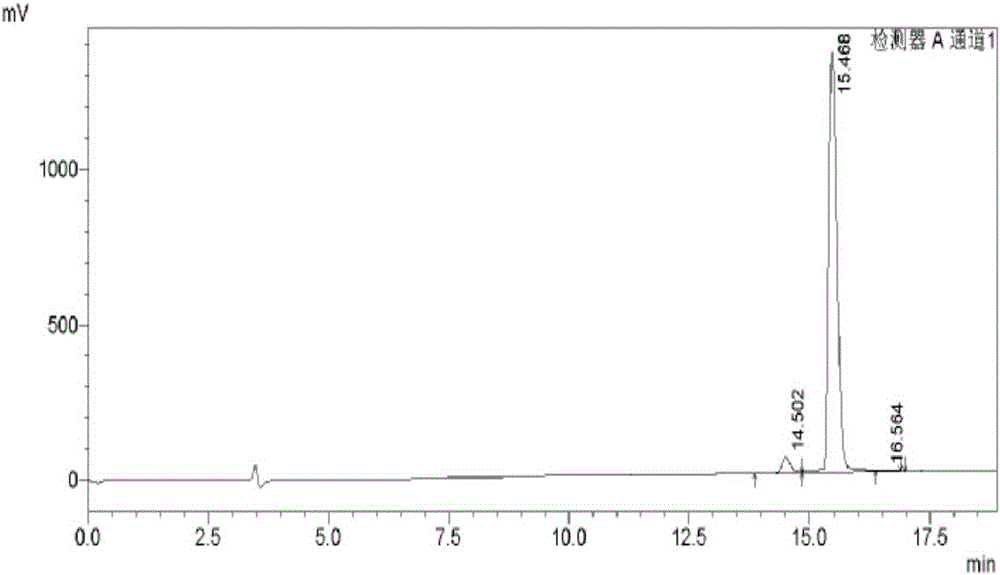
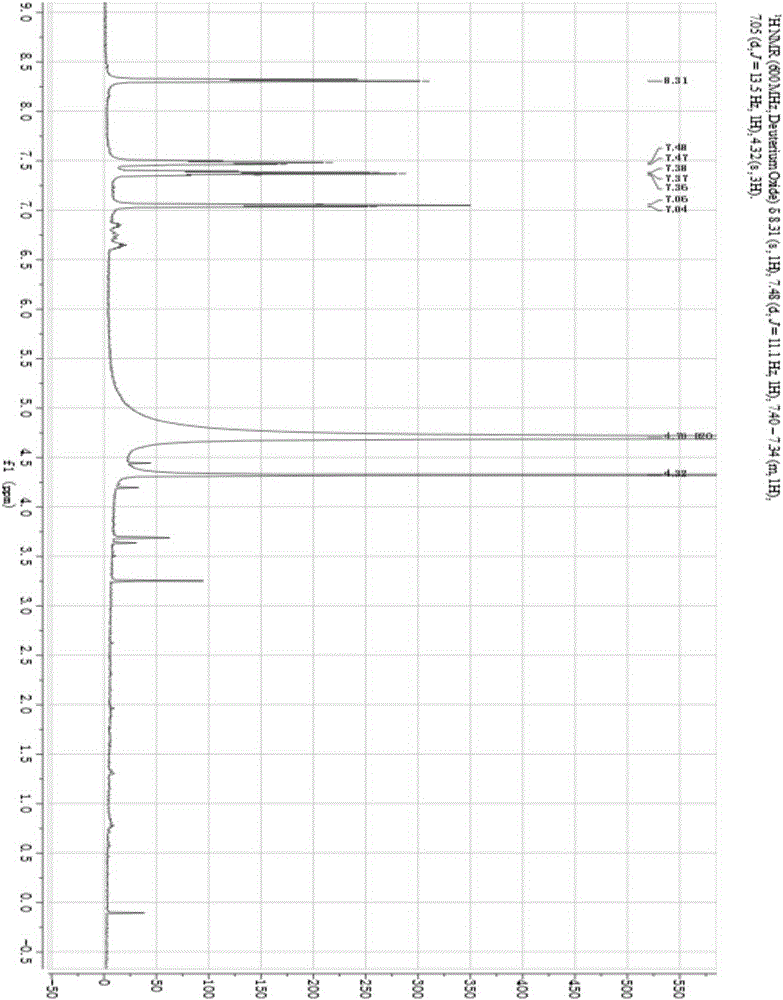
[0054] Preparation and Structure Identification of 1-Methoxy-4,5-Dihydroxyisoquinoline from Centipede
[0055] A. water extraction method and alcohol extraction method prepare centipede extract:
[0056] (1) Grinding the dry body of the centipede to obtain centipede powder, adding 10 times the volume of the centipede powder (5-10 times the volume is acceptable, preferably 10 times in this embodiment, and the same reasoning behind), and then performing ultrasonic crushing;
[0057] (2) Freezing and centrifuging the homogenate, taking the supernatant, and freeze-drying to obtain the centipede aqueous extract precipitation;
[0058] (3) Take the precipitate obtained in step (2), add 55% (volume fraction, the same reason behind) ethanol homogenization of 10 times the volume of the precipitate, then leaching at 4°C, and freeze and centrifuge the leaching solution again , take the supernatant, freeze-dry to obtain centipede 55% ethanol extract;
[0059] (4) Take the precipitate ob...
Embodiment 2
[0085] The step of extracting active component in the present embodiment is basically the same as embodiment 1, and difference is: 1) during water extraction, the PBS solution volume that adds is 5 times of centipede powder volume, during alcohol extraction, the volume fraction of alcoholic solution is 45%; 2) When adopting Sephadex gel chromatography to separate and purify centipede ethanol extract, centipede ethanol extract is configured to 20mg / mL, mobile phase is 50% ethanol, loading volume is 8mL, and flow rate is 0.6mL / min.
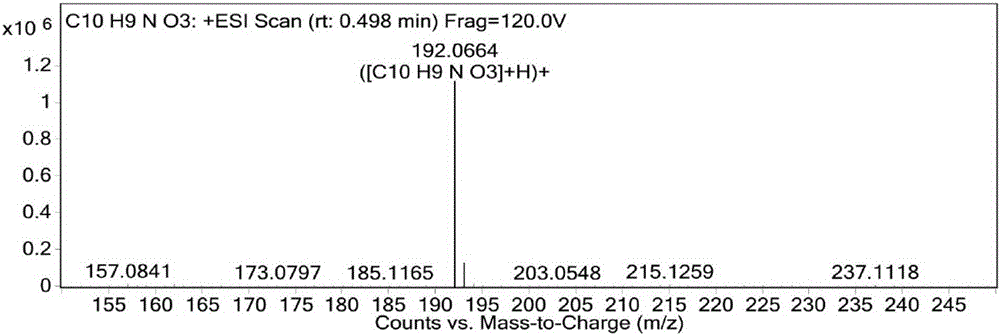
[0086] The result of this example is the same as Example 1, and the active substance finally obtained is identified as 1-methoxy-4,5-dihydroxyisoquinoline (1-methoxy-4,5-diolisoquinoline) by structure, and the molecular formula is C 10 h 9 NO 3 , the molecular weight is 191.1, and the structural formula is:
[0087]
Embodiment 3
[0089] The step of extracting active component in the present embodiment is basically the same as embodiment 1, and difference is: 1) during water extraction, the PBS solution volume that adds is 8 times of centipede powder volume, during alcohol extraction, the volume fraction of alcoholic solution is 65%; 2) When adopting Sephadex gel chromatography to separate and purify centipede ethanol extract, centipede ethanol extract is configured to 35mg / mL, mobile phase is 30% ethanol, sample volume is 6mL, and flow rate is 1.5mL / min.
[0090] The result of this example is the same as Example 1, and the active substance finally obtained is identified as 1-methoxy-4,5-dihydroxyisoquinoline (1-methoxy-4,5-diolisoquinoline) by structure, and the molecular formula is C 10 h 9 NO 3 , the molecular weight is 191.1, and the structural formula is:
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com